Immunotherapy has become an important therapy in the management of advanced skin cancer. Monotherapy with anti-programmed cell death protein 1 (anti-PD-1) is currently the first-line therapy for advanced cutaneous squamous cell carcinoma (cSCC) in patients ineligible for curative surgery and/or radiotherapy. Cemiplimab has shown an objective response rate of 47.5% with a response duration of more than 6 months in 57% of responding patients.1 Immune checkpoint inhibitors (ICI) modify the tumor immune microenvironment leading not only to anti-tumor responses but also immune-related adverse effects (irAEs).2,3 Rheumatic irAEs have been reported in approximately 10% of patients on ICI and there are currently no established guidelines for their therapeutic management, except for recommendations that include avoiding doses >10mg/day of prednisone or equivalent.4,5
We present three cases of advanced cSCC ON anti-PD-1 that presented arthralgia and/or myalgia which were successfully treated with hydroxychloroquine.
- -
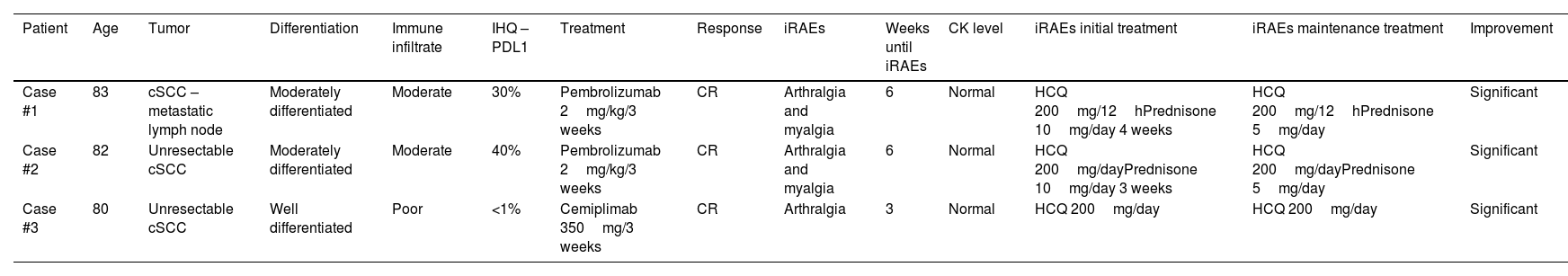
Case #1: An 83-year-old woman with cSCC in the right cheek and unresectable lymph node metastasis refractory to radiation therapy received pembrolizumab 2mg/kg every 3 weeks. The patient achieved complete clinical and radiological response after 6 cycles and maintained this response after 17 cycles. However, on dose #3, she presented with grade 2 arthromyalgia. Lab test results revealed a C-reactive protein (CRP) level of 33.5U/L (0–5mg/L), an erythrocyte sedimentation rate (ESR) of 21mm/h (1–20mm/h), and creatin-kinase (CK) levels of 41U/L (29–168U/L). At the onset of symptoms, she was prescribed 10mg/day of prednisone and oral hydroxychloroquine 200mg/12h which led to complete symptom relief within 3 weeks (Table 1). Then, a maintenance treatment of 5mg/day of prednisone and hydroxychloroquine 200mg/12h was employed.
Table 1.Demographic features, cancer types, immunotherapy and rheumatic immune-related adverse events (irAEs).
Patient Age Tumor Differentiation Immune infiltrate IHQ – PDL1 Treatment Response iRAEs Weeks until iRAEs CK level iRAEs initial treatment iRAEs maintenance treatment Improvement Case #1 83 cSCC – metastatic lymph node Moderately differentiated Moderate 30% Pembrolizumab 2mg/kg/3 weeks CR Arthralgia and myalgia 6 Normal HCQ 200mg/12hPrednisone 10mg/day 4 weeks HCQ 200mg/12hPrednisone 5mg/day Significant Case #2 82 Unresectable cSCC Moderately differentiated Moderate 40% Pembrolizumab 2mg/kg/3 weeks CR Arthralgia and myalgia 6 Normal HCQ 200mg/dayPrednisone 10mg/day 3 weeks HCQ 200mg/dayPrednisone 5mg/day Significant Case #3 80 Unresectable cSCC Well differentiated Poor <1% Cemiplimab 350mg/3 weeks CR Arthralgia 3 Normal HCQ 200mg/day HCQ 200mg/day Significant F: female; M: male; CR: complete response; IHQ: immunohistochemistry; CK: creatine kinase; HCQ: hydroxychloroquine.
- -
Case #2: An 82-year-old man with recurrent cSCC affecting the right inner canthus, who was ineligible for surgery or radiotherapy on pembrolizumab 2mg/kg every 3 weeks. He presented clinical and radiological response on cycle #5, which was maintained after 11 cycles. However, on cycle #3, he developed grade 2 arthromyalgia. Lab test results showed a CFP level of 60.5mg/L (0–5mg/L), an ESR of 32mm/h (1–20mm/h) and CK levels within the normal range. Symptoms resolved with a 1-month initial regimen of prednisone 10mg/day and oral hydroxychloroquine 200mg/day (Table 1), after which the treatment was down-titrated to 5mg/day of prednisone and hydroxychloroquine 200mg/day as maintenance therapy.
- -
Case #3: An 80-year-old man with locally advanced recurrent cSCC on his right wrist, refractory to radiotherapy on a 2-year regimen of cemiplimab 350mg every 3 weeks achieved sustained complete clinical response after treatment discontinuation. On cycle, he presented with grade 2 arthralgia. Blood test results revealed CRP levels of 9.5mg/L (0–5mg/L), an ESR of 46mm/h (1–20mm/h) and normal CK levels. He was successfully treated with hydroxychloroquine 200mg twice-daily (Table 1).
In all three cases, the rheumatoid factor and citrullinated peptide antibody tests were negative.
Through these three cases we aim to highlight the effectiveness of hydroxychloroquine in the management of musculoskeletal side effects or rheumatic irAEs. As far as we know, this is the first study ever conducted to show complete resolution using hydroxychloroquine.
Hydroxychloroquine is used in the management of various rheumatological, immunological and infectious diseases. In addition to its well-known anti-inflammatory, immunomodulatory, anti-infective, anti-thrombotic and metabolic effects6 it also exhibits potent antiproliferative and antimutagenic properties.7 Furthermore, it is usually considered a safe treatment with few adverse effects. Retinopathy, although concerning, is rare when administered at doses <5mg/kg/day7 and is potentially reversible.
The actual prevalence of rheumatic irAEs is estimated to be around 10% but there are limited studies reporting prevalence of this disease, likely due to their relatively mild nature and sometimes lacking clinical suspicion.4,8 The most common rheumatic irAEs include arthralgia, myalgia, arthritis and polymyalgia rheumatic-like syndrome.5 Some studies indicate that the prevalence of arthralgia ranges from 1% up to 43% vs 1% up to 7% of arthritis.5 These side effects are more commonly associate with anti-PD-1 drugs or combined ICI.9 The estimated prevalence of arthralgia with pembrolizumab is estimated at 9–12% but may run unnoticed. Arthralgia typically affects large joints symmetrically9 and tends to occur around the third or sixth month after the beginning of immunotherapy. Serological markers such as rheumatoid factor, citrullinated peptide antibody or CK levels are generally not elevated.9
Managing irAEs can often be achieved without discontinuing immunotherapy, but it requires maintaining the prednisone dosage <10mg/day (or equivalent) to avoid compromising its efficacy.4 Some irAEs can persist despite treatment discontinuation. While the Cancer Immunotherapy Society recommends the use of disease-modifying antirheumatic drugs, hydroxychloroquine is not currently mentioned as a potential corticosteroid-sparing agent.6
In conclusion, whether used as monotherapy or as an adjuvant therapy hydroxychloroquine appears to be a safe and effective option to address musculoskeletal symptoms without compromising the efficacy of immunotherapy. Further studies are needed to validate its role in managing these patients.
Conflict of interestsThe authors state that they have no conflict of interests.
The lead author had full access to all the data in the study and takes full responsibility for the integrity of data and the accuracy of data analysis. The undersigned declared no conflicts of interest, financial activities or any other relationships or activities that readers could perceive to have influenced the study, or give the appearance of having influenced the content of the submitted work. This study received no funding whatsoever.