Mastocytosis encompasses a spectrum of disorders in which different organs and tissues are affected by the clonal expansion of mast cells. The skin is one of the most frequently affected organs. The clinical manifestations of mastocytosis are linked to the release of proinflammatory mediators, and the impact of this disorder on patient quality of life has been described in various studies. The Mastocytosis Quality of Life Questionnaire (MC-QoL), which was recently developed in Germany and now also exists in English, is an important tool for evaluating the psychosocial impact of this disease.
ObjectiveTo create a Spanish version of the MC-QoL that was culturally equivalent to the original German questionnaire.
Material and methodsThe adaptation process, which involved forward translation, cognitive interviews, and back translation, was conducted in accordance with the principles of good practice for the translation and cultural adaptation of patient-reported measures of the International Society for Pharmacoeconomics and Outcomes Research. The MC-QoL contains 27 items in 4 domains: symptoms, emotions, social life/functioning, and skin.
ResultsThe first version of the Spanish questionnaire, obtained by direct translation from German, was tested in cognitive interviews, after which 3 items were modified to make them easier to understand. The German back translation of the Spanish questionnaire was analyzed by the authors of the original MC-QoL, who modified 1 item they considered to have lost specificity in the adaptation process. The definitive Spanish MC-QoL was then produced following minor modifications agreed on with the German authors.
ConclusionsWe have produced a cultural adaptation of the MC-QoL in Spanish that can be used in routine clinical practice to obtain a more complete picture of the impact of mastocytosis on patient quality of life.
Las mastocitosis son un grupo de proliferaciones clonales de mastocitos que pueden afectar a distintos órganos y tejidos, siendo la piel uno de los implicados con mayor frecuencia. La clínica está relacionada con la liberación de mediadores proinflamatorios. Diversos estudios han señalado la afectación en la calidad de vida de estos pacientes. El reciente desarrollo en Alemania de un cuestionario específico sobre calidad de vida en pacientes con mastocitosis (Mastocytosis Quality of Life questionnaire [MC-QoL]), con una versión en inglés, supone un paso significativo en la valoración del impacto psicosocial.
ObjetivoRealizar la adaptación transcultural al castellano del MC-QoL, garantizando su equivalencia con la versión original.
Material y métodosMetodología de traducción directa, entrevistas cognitivas y traducción inversa, siguiendo los principios de buena práctica de la International Society for Pharmacoeconomics and Outcomes Research. El MC-QoL incluye 27 preguntas estructuradas en 4 dominios: síntomas, emociones, funcionamiento/vida social y sintomatología cutánea.
ResultadosLa primera versión del cuestionario en español, obtenida por traducción directa de la versión en alemán, se sometió a entrevistas cognitivas, modificándose 3 preguntas para hacerlas más comprensibles. Posteriormente, los autores originales modificaron una pregunta de la versión obtenida por retrotraducción al considerar que había perdido especificidad. Tras mínimas modificaciones en consenso con los autores originales, se obtuvo la versión definitiva en castellano.
ConclusiónSe obtiene la adaptación transcultural en castellano de un cuestionario de calidad de vida específico para pacientes con mastocitosis, que podrá ser utilizado en la práctica clínica para una valoración más completa de este grupo de pacientes.
Mastocytosis encompasses a heterogeneous group of conditions characterized by clonal proliferation of mast cells. Various organs and tissues can be affected. Mastocytosis is rare, as indicated by an estimated prevalence of 9 to 13 adult cases per 100 000 population for the indolent systemic form.1,2 Although mastocytosis is more frequent in the first decade of life and between the second and fifth decades, it can manifest at any age and affects both sexes similarly.3,4
Signs and symptoms (such as pruritus, erythema, palpitations, headache, abdominal pain, diarrhea, hypotension, irritability, and anaphylaxis among others) are related to the release of proinflammatory mediators that infiltrate organs and tissues; other associated hematologic conditions may also be present.3,5–10 Skin involvement is the most common sign — present in all pediatric cases and 80% of adult cases — and often provides the key to diagnosing the disease.3,9,11
Although clinical signs are heterogeneous and variable, the condition develops slowly in most patients. Nonetheless, several studies have reported a significant impact on patients’ quality of life (QoL),12 as a consequence of both the symptoms themselves and the need for continuous treatments to alleviate or prevent them.13 QoL improvement is a main objective in managing mastocytosis, and results obtained with health related QoL questionnaires have become increasingly important in both routine clinical practice and the conduct of clinical trials. Such questionnaires provide information from the patient’s perspective and prevent loss of useful information noted during the clinical evaluation of individuals.14
Health related QoL tools for specific diseases or symptoms provide more solid evidence than generic questionnaires. A specific tool for mastocytosis was unavailable, however, until the German group of Siebenhaar et al15 developed and validated the Mastocytosis QoL (MC-QoL) questionnaire for adults with skin manifestations of indolent systemic mastocytosis. (See online supplementary material, Appendix, Fig. 1, the German Version, and Fig. 2, the American English version.) The MC-QoL is a significant step forward in the evaluation of the psychological and social impact of this disease.
Our aim was to translate and culturally adapt the MC-QoL to peninsular Spanish, preserving its equivalence to the original version, so that it could be used in routine clinical practice in Spain.
Material and MethodsThe MC-QoL is composed of 27 questions in 4 domains: general symptoms, emotions, social life/functioning, and skin symptoms. Each question has 5 possible answers.
We followed published guidelines when planning the translation and adaptation process.16–20 Particularly important were the principles of good practice by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).17
The method involved translation and back translation in the following stages: translation of the original German questionnaire to Spanish by a bilingual translator (native language, peninsular Spanish), patient interviews for cognitive debriefing, and finally back translation of the Spanish version to German by another bilingual translator (native language, German) for review by the authors of the original German questionnaire (Fig. 1).
The working group was made up of 2 Spanish dermatologists, a German dermatologist expert in mastocytosis, 2 professional bilingual translators, and an expert in the validation of questionnaires.
Interviews for cognitive debriefing were conducted with patients in order to ensure their correct understanding of concepts in the questionnaire. All 6 patients interviewed were being treated for mastocytosis in our hospital. They had different sociodemographic profiles and were diagnosed with either indolent systemic or cutaneous mastocytosis. All diagnoses were confirmed by bone marrow or skin biopsy. Three were men and 3 were women. Their mean age was 45.7 years (median, 49 years). Two had cutaneous mastocytosis and 4 had the indolent systemic form.
The principal investigator explained the type of study and its objectives in detail to all 6 patients and described the questionnaire. The patients gave their written informed consent to participation. Verbal probing was used during the cognitive debriefing sessions,19–22 and the patients followed a structured protocol, as follows: they read all sections of the questionnaire aloud, starting with the instructions and each question one by one, answering them as they went along. During this process, the patients were asked to explain in their own words what they had just read and to point out parts of the text that seemed confusing or difficult to interpret. The participants’ expressions, comments, and even their gestures were noted in case they might indicate where the questionnaire was difficult to understand. They were also asked whether they might have expressed something differently or if they could have understood something better if different phrasing had been used. Their answers were carefully and literally noted so that the working group could evaluate them afterwards. Interviews lasted about 20 to 30 minutes.
After review and protocolized discussion of the results, the working group produced a second draft of the questionnaire for professional back translation to German and review by the original authors. A third Spanish version was created based on the original authors’ comments and suggestions and once again back translated to German for another evaluation. One question required further revision and a few small changes were made to produce the fourth and final version of the adapted Spanish MC-QoL. (See online supplementary material, Appendix, Fig. 3, the final Spanish version.)
ResultsSeveral revisions were made as a result of the cognitive interviews, during which the patients revealed their comprehension difficulties and the interviewer recorded their suggestions.
First, small changes were made in 2 sentences in the general instructions.* (See online supplementary material, Appendix B, Figs. 1, German, and 2, English.) Several words were changed in the drafted sentence saying, “Please read each question with attention and select from among the 5 options the one that is most appropriate for you.” The Spanish draft was changed in the final version to, “Please read each question carefully (in Spanish, the word was detenidamente, implying attending to detail, not rushing) and select from among 5 options the one that best corresponds to your situation.” The words or phrases shown in italics mark the points of revision. These changes were made because the literal translation from German to Spanish placed emphasis on elements that did not help the reader. Also changed, in the interest of easier comprehension, was the first-version sentence, “Don’t think too much (in Spanish, demasiado) about your answer and don’t forget to answer all the questions.” The revised version was “Please don’t think much about your answer and make sure you answer all the questions.”
Minimal changes were also made in the 5 possible answers for some of the questions (eg, from un poco [“a little”] to poco [“little”] and from muchísimo [“a great deal” or “very much”] to extremadamente [“extremely”] given that these modifications made more sense in relation to the Spanish wording of the questions.
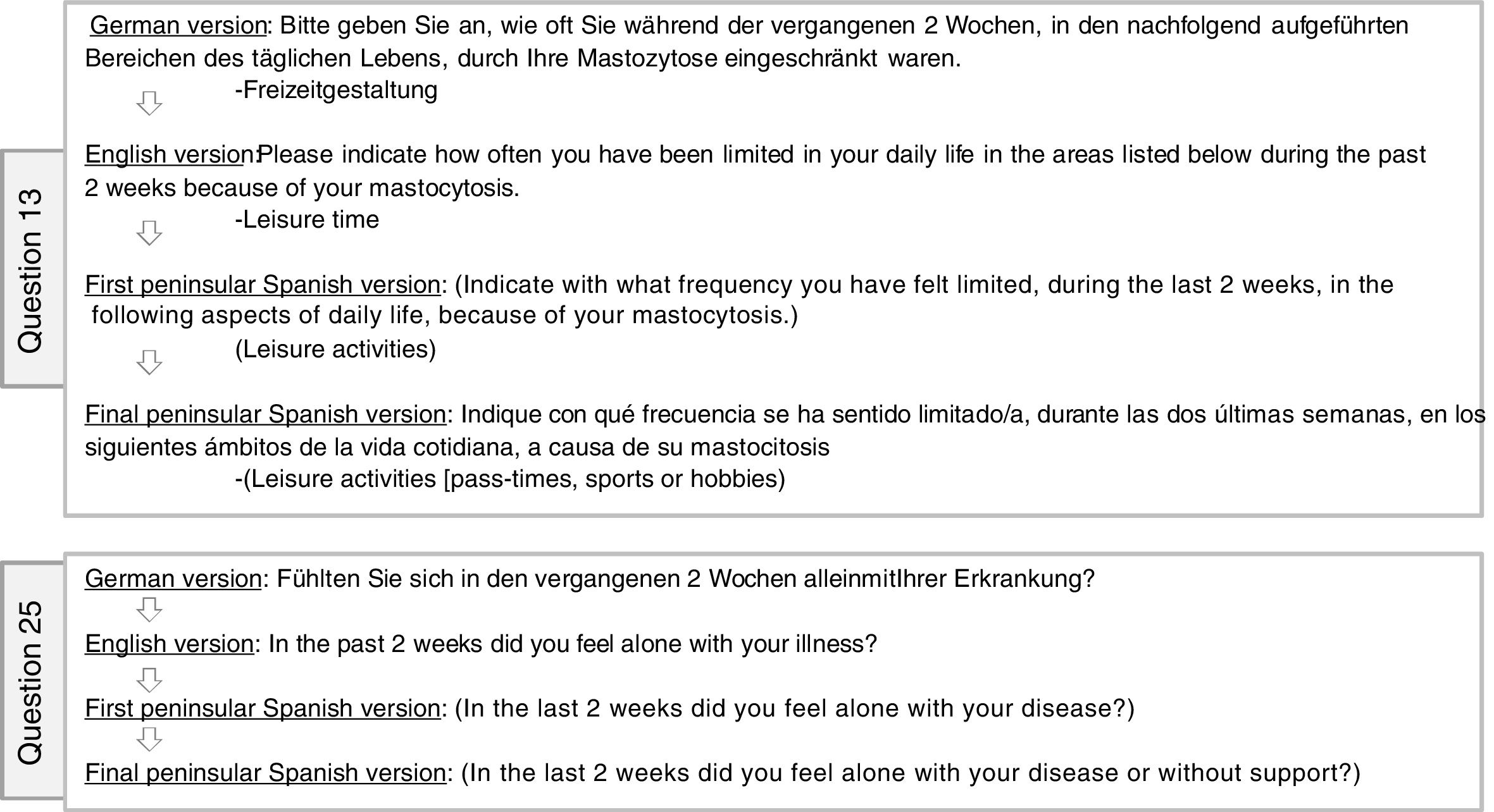
Three of the 27 questions (numbers 13, 18, and 25) were revised. Question 13 required the addition of some examples to enable better patient understanding, as it seemed vague without them (Fig. 2). Question 25, which asked whether the patient se sintió solo/a (“felt lonely”) was not entirely clear to some patients, so a Spanish paraphrase of the concept, o sin apoyo (“or without support”), was added to make the concept more concrete and easier to grasp (Fig. 2). Finally, question 18 was the most problematic, and the only one that was revised by the original version’s authors after back translation. The phrase ha notado la carga de los síntomas (“have noticed the burden of the symptoms”) was changed because it had been translated too literally and the Spanish word carga (“burden”) did not seem to be semantically equivalent to its corresponding words in German or English, creating a comprehension problem for patients. A small change — the addition of o el peso (“or the weight”) — failed to reflect the main purpose of the question, leaving it difficult to understand correctly. Therefore, in collaboration with the authors of the original German questionnaire, an attempt was made to explain the concept — as feeling agobiado o sobrecargado (“overwhelmed or overburdened”) in Spanish (Fig. 3) rather than just look for target-language synonyms to translate the concept of “burden” by itself. However, the authors of the original still felt the specificity of the original German phrase, which refers to emotional burden, had been lost. Finally, consensus was reached among all parties involved, and a minimal change was made, with the addition of the word emocionalmente (“emotionally”) to the Spanish question (Fig. 3). Thus, agreement on the final version of the questionnaire in Spanish was reached. (See online supplementary material, Appendix B, Fig. 3, the final Spanish version.) The format of the questionnaire did not have to be modified, as it seemed simple and intuitive to all the patients interviewed.
Changes made in questions 13 and 25 of the Mastocytosis Quality of Life (MC-QoL) questionnaire. (Translator’s note: The translations in this figure adhere as closely as possible to the Spanish phrasing so that the English-language reader of this article can intuit the reasons why the Spanish revisions were necessary. Readers interested in the validated cultural adaptation/translation of the MC-QoL to English must consult the online supplement [Fig. 2] for this article).
Changes made in question 18 of the Mastocytosis Quality of Life questionnaire (MC-QoL). (Translator’s note: The translations in this figure adhere as closely as possible to the Spanish phrasing so that the English-language reader of this article can intuit the reasons why the Spanish revisions were necessary. Readers interested in the validated cultural adaptation/translation of the MC-QoL to English must consult the online supplement [Fig. 2] for this article).
A wide range of tools are currently available for evaluating and quantifying the impact of different diseases on QoL, and dermatology makes use of both general and disease-specific ones. Even though the literature on mastocytosis shows that a large number of patients with this disease find that their daily life is affected by symptoms,12,15 before the MC-QoL was developed no specific tool was available. This questionnaire therefore represents a significant step forward in the clinical evaluation of patients with mastocytosis.
The MC-QoL was developed and validated in Germany, in patients diagnosed with cutaneous or indolent systemic mastocytosis. The American English translation was validated and published at the same time. The MC-QoL is a simple 27-item questionnaire that can be administered quickly. Each item is answered by choosing from among 5 options, each of which counts from 0 to 4 points toward a score. The tool has 4 domains related to different aspects of the disease and patients’ daily life: general symptoms, emotions, social life/functioning, and skin symptoms. Domain and total scores are calculated by adding up points for individual answers and then expressing the result on a scale from 0 to 100, where higher scores indicate better QoL.15
The validity and reliability of the new tool, in terms of both total and domain scores, were high. Nearly all domain scores had high internal validity statistics. The exception was skin symptoms, which had slightly lower internal validity. Convergent validity was also satisfactory, as scores were found to correlate highly with scores on the Dermatology Life Quality Index, the Short Form-12, the ItchyQoL questionnaire, and the EuroQoL-visual analog scales, among others. The authors who developed the MC-QoL questionnaire also reported that the correlations were strong between the different domain scores and the specific questionnaires used for comparison.15 Multiple regression analysis showed that disease duration was a significant factor in loss of QoL, whereas age, sex and skin involvement were not.
Soon after the development of the MC-QoL, a group in the Netherlands developed two specific questionnaires — the Mastocytosis Quality-of-Life Questionnaire (MQLQ) and the Mastocytosis Symptom Assessment Form (MSAF) — for use in patients with indolent systemic mastocytosis.23 Results of administering the MSAF showed that fatigue and sleep difficulties were the most severe symptoms. MQLQ results showed that fear of anaphylaxis had the greatest impact on QoL. These 2 questionnaires have since been used in several published studies on the psychosocial impact of mastocytosis. The results are consistent with previous reports in the literature. A recent review of patients’ perceptions of changes related to mastocytosis24 found that they reported a moderate to severe decrease in QoL, a negative impact on affect in daily life, and a psychological impact in over 60% to 70% of cases.12,23–26 Among the feelings most often mentioned were sadness, worry, frustration, embarrassment caused by visible skin lesions, fear for the future, and a feeling of being a burden to others.24 Another study of 7 patients with indolent systemic mastocytosis was recently published by a group in Denmark.27 Based on data from semistructured interviews with those patients, the authors reported a strong impact of the disease on the patients’ daily lives, particularly in social relationships, self-image, and sexuality. Symptoms restricted and complicated their participation in various activities, and they felt constant fear of an anaphylactic reaction.
Our transcultural adaptation of the MC-QoL facilitates the clinical use of this questionnaire with Spanish-speaking patients in Spain. We used a rigorous, systematic method that ensures the Spanish version of the questionnaire is equivalent to the original. This method has been used repeatedly in Europe to prepare measurement tools for clinical and research applications.16–19 We suggest that research to further validate the MC-QoL in use should be undertaken in the future in the interest of confirming its sensitivity, specificity, validity and reliability in patients in various clinical settings. Such research would require coordinated multicenter studies with a common aim.
ConclusionWe now have a culturally adapted peninsular Spanish version of the MC-QoL to use in clinical practice and research to measure the impact of mastocytosis on QoL. This tool can help us gain deeper understanding of the needs, experiences, and fears of patients with mastocytosis and facilitate more comprehensive management of the condition.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Translator’s note: The translations in this section and in Figs. 2 and 3 adhere as closely as possible to the Spanish phrasing so that the English-language reader of this article can intuit the reasons why the Spanish revisions were necessary. However, readers interested in the validated cultural adaptation/translation of the MC-QoL to English must consult Figure 2 of the online supplement (Appendix B) for this article.
Please cite this article as: Bertolín-Colilla M, Garin-Boronat O, Siebenhaar F, Maurer M, Pujol RM, Giménez-Arnau AM. Adaptación transcultural del cuestionario Mastocytosis Quality of Life questionnaire (MC-QoL) del alemán al castellano. Actas Dermosifiliogr. 2020;111:243–248.




![Changes made in questions 13 and 25 of the Mastocytosis Quality of Life (MC-QoL) questionnaire. (Translator’s note: The translations in this figure adhere as closely as possible to the Spanish phrasing so that the English-language reader of this article can intuit the reasons why the Spanish revisions were necessary. Readers interested in the validated cultural adaptation/translation of the MC-QoL to English must consult the online supplement [Fig. 2] for this article). Changes made in questions 13 and 25 of the Mastocytosis Quality of Life (MC-QoL) questionnaire. (Translator’s note: The translations in this figure adhere as closely as possible to the Spanish phrasing so that the English-language reader of this article can intuit the reasons why the Spanish revisions were necessary. Readers interested in the validated cultural adaptation/translation of the MC-QoL to English must consult the online supplement [Fig. 2] for this article).](https://static.elsevier.es/multimedia/15782190/0000011100000003/v1_202005050702/S1578219020300305/v1_202005050702/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Changes made in question 18 of the Mastocytosis Quality of Life questionnaire (MC-QoL). (Translator’s note: The translations in this figure adhere as closely as possible to the Spanish phrasing so that the English-language reader of this article can intuit the reasons why the Spanish revisions were necessary. Readers interested in the validated cultural adaptation/translation of the MC-QoL to English must consult the online supplement [Fig. 2] for this article). Changes made in question 18 of the Mastocytosis Quality of Life questionnaire (MC-QoL). (Translator’s note: The translations in this figure adhere as closely as possible to the Spanish phrasing so that the English-language reader of this article can intuit the reasons why the Spanish revisions were necessary. Readers interested in the validated cultural adaptation/translation of the MC-QoL to English must consult the online supplement [Fig. 2] for this article).](https://static.elsevier.es/multimedia/15782190/0000011100000003/v1_202005050702/S1578219020300305/v1_202005050702/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



