Current guidelines recommend omalizumab as the second-line therapy for chronic urticaria (CU) after failed high-dose antihistamines (AH) (3 or 4 times the standard dose).1 However, it has not been defined whether these AH should be maintained while on omalizumab, what the optimal dosage of AH is, and whether they offer any real benefits to the patient.
The aim of this study was to analyze the use of AH before, 3 and 6 months after starting omalizumab, and evaluate the differences between prescribed and consumed AH. Therefore, we conducted a single-center, retrospective, observational study with a cohort of 48 adult patients diagnosed with CU who had been on omalizumab for, at least, 6 months.
To determine drug use by the patients, we consulted the dispensation of these drugs in the electronic prescription system of the Community of Madrid, Spain. Patients were classified based on urticaria activity using the Urticaria Activity Score over 7 days (UAS7) the Urticarial Control Test (UCT), and according to the AH doses. The chi-square test and the Mann-Whitney U test were used to analyze the study variables. Furthermore, the McNemar symmetry test was used to compare prescribed and consumed AH figures. The software used was the IBM Statistical Package for the Social Sciences (SPSS).
A total of 32 out of the 48 patients included in this study (66.7%) were women and the mean age was 49 years. A total of 23% of the patients showed autoimmune comorbidities (with hypothyroidism being prominent) and 33% stigmata of atopic constitution. Patients with spontaneous CU (66% overall), inducible urticaria (IU) (11% overall), and those with spontaneous CU and IU (23%) were studied together.
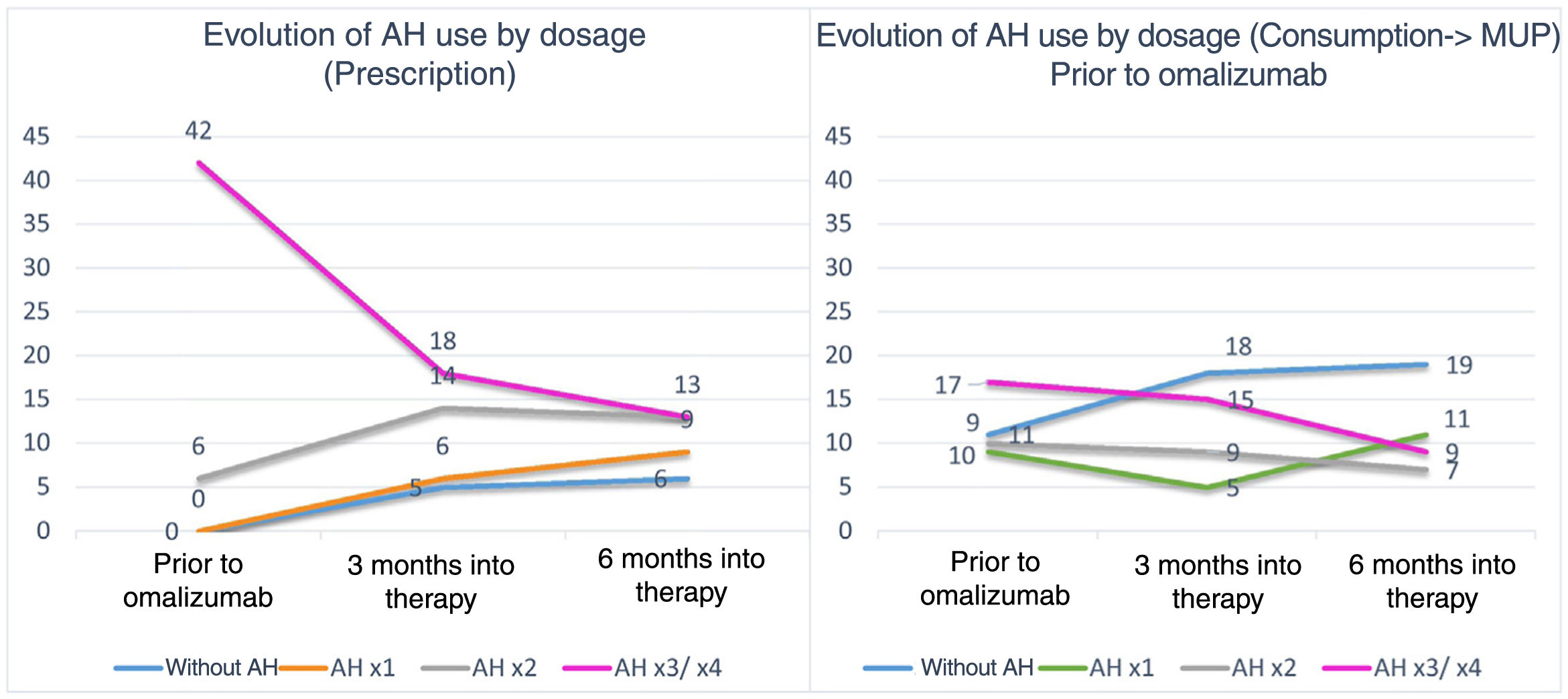
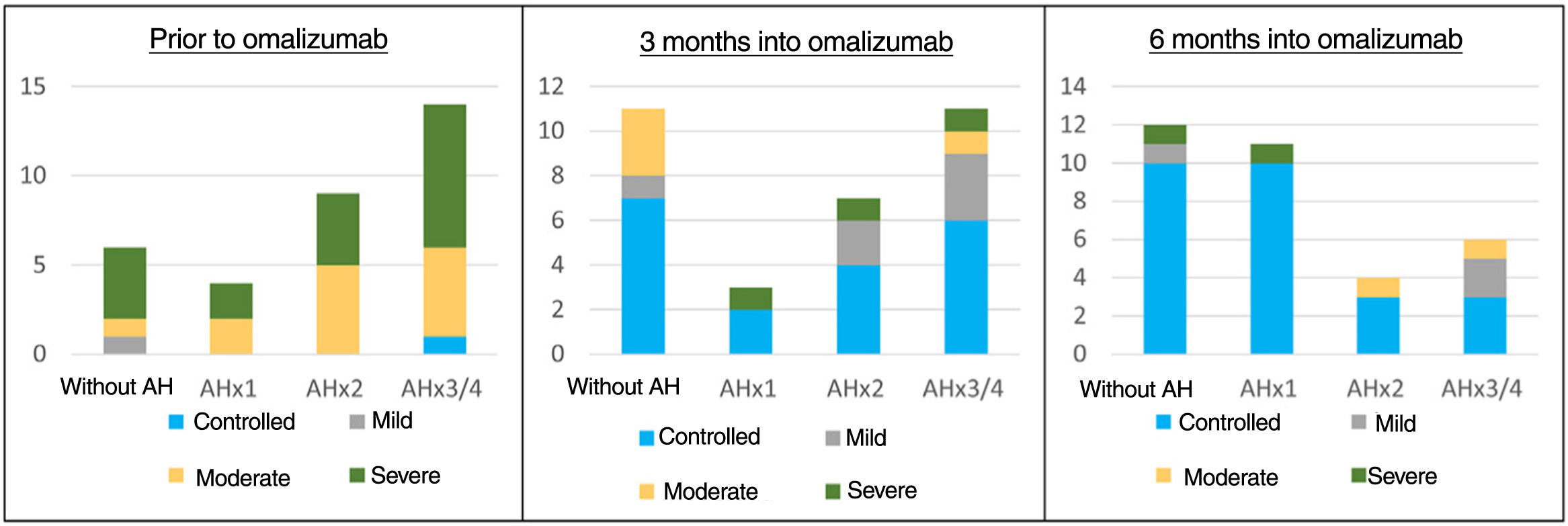
We saw that prior to starting omalizumab, high-dose AH were prescribed in up to 94% of the patients with CU. Three and 6 months into treatment, the prescription was more heterogeneous (figure 1). Regarding the actual use of AH, we saw that prior to starting omalizumab, only 42% of the patients were on high-dose AH (figure 1). McNemar's test revealed significant differences between the number of patients to whom each dose was prescribed and the number actually taking that dose, indicating low treatment adherence. To study the impact of AH use on the disease, a correlation between disease control and actual AH use was established (figure 2). No statistically significant differences were found determining greater urticaria control based on the AH dose used.
Shows the number of patients on the vertical axis. Each month of the study is represented on the horizontal axis. Colored lines show the evolution of the different doses of prescribed (left graph) or consumed AH (right graph) by the patients during the study period. The numbers on the lines indicate the exact number of patients who consumed or were prescribed that dose of AH during that studied month. After performing McNemar's test, significant differences were seen between prescribed and consumed AH figures, indicating low treatment adherence.
AH, antihistamines.
Shows the absolute number of patients (vertical axis) consuming each dose of AH (transverse axis) according to the month of treatment studied. Patients are classified based on urticaria activity (represented by colored segments in the columns of each graph), considering controlled CU if UAS7 is 0-6, mild urticaria if between 7 and 15, moderate if between 16 and 27, and severe if between 28 and 42. Only patients diagnosed with spontaneous CU were included in this graph. It becomes obvious that longer biologic regimens of treatment are associated with better disease control, and a lower consumption of AH.
AH, antihistamines; CU, chronic urticaria; UAS7, Urticaria Activity Score over 7 days.
This research shows uniformity regarding prescription among dermatologists prior to starting biologics (reflecting the clinical guidelines), a uniformity that is not maintained after the start of omalizumab. There is also no consensus in the literature on what AH regimen should be prescribed after omalizumab has been started. Some studies argue that AH consumption should be on-demand, intensifying AH in case of worsening symptoms, with good results controlling flare-ups.2 Others maintain that the AH dose should be the dose used before starting the biologic therapy, i.e., high doses of AH,3,4 while others suggest the possibility of stopping AH right from the beginning of biologic therapy. In such cases, as in our research, it has been demonstrated that there is no clear benefit from AH during anti-IgE therapy and it is argued that if a benefit was never to be obtained from these drugs, the best thing to do is discontinue them.5–7
On the other hand, there is a significantly low treatment adherence on the patients’ end, which poses the question of what the real cause of this low adherence may be. Melé et al. published a study analyzing AH use during omalizumab treatment through a survey. Among the reasons justifying low AH adherence, a good response to the biologic drug was the most common cause.8
Limitations of this study include its retrospective design, the distinction between spontaneous CU and IU, and the sample size. However, as an advantage, objective data obtained from the prescription system of the Community of Madrid were handled.
In conclusion, it appears that AH use may not be necessary to control CU in patients on omalizumab if the patient is already under control with the biologic drug. It seems necessary to find a pattern in the AH guidelines while on omalizumab and emphasize therapeutic adherence.
Conflicts of interestNone declared.