The new European guidelines on atopic dermatitis (AD) have recently been published, covering the main revolution in this field: the new systemic drugs. In adulthood, baricitinib, cyclosporine, dupilumab, upadacitinib, and tralokinumab are drugs with a strong grade of recommendation, while in childhood and adolescence they are limited to cyclosporine, dupilumab, and upadacitinib. The main question that needs to be answered is: can we select a specific drug based on the patient's characteristics?
AD is a complex disease of a wide heterogeneity regarding clinical phenotypes, age, ethnic origin, and severity. Its origin involves dysregulated immune response, impaired skin barrier, and dysbiosis with Staphylococcus aureus overgrowth. Although, initially, it was believed to be a disease caused solely by the Th2 immunological pathway, other pathways involved such as Th1, Th17, or Th22 have been discovered, with different relevance depending on the disease stage, age group, and ethnicity.1,2
This wide phenotypic and immunological spectrum has led to the search for biomarkers to objectively determine screening, diagnosis, prognosis, monitoring of AD severity, and associated comorbidities. However, the definition of a biomarker is not uniformly accepted. The European Medicines Agency defines it as “a detectable molecule in blood, fluids, or body tissues that allows the monitoring of a bodily or pathological process”. Its American counterpart, the U.S. Food and Drug Administration, expands this concept to “any measurable characteristic that acts as an indicator of a normal biological process, a pathological process, or a response to an exposure or procedure, including therapeutic interventions”. Therefore, not only molecules are included, but also histopathological or radiographic data, and physiological characteristics.3–5One of the most widely studied biomarkers in the management of AD is mutations in the filaggrin gene. This gene encodes essential proteins for the proper functioning of the skin barrier and has been proposed as a screening biomarker and predictor of severe and early forms of the disease and possible increased risk for developing asthma along with immunological changes.3–5 Based on the variety of molecules involved in the origin of AD, other authors have proposed categorizing patients into molecular groups—the so-called endotypes—to select targeted therapies vs the impaired leucines and chemokines in those patient groups and then be able to increase the therapeutic efficacy.1,2
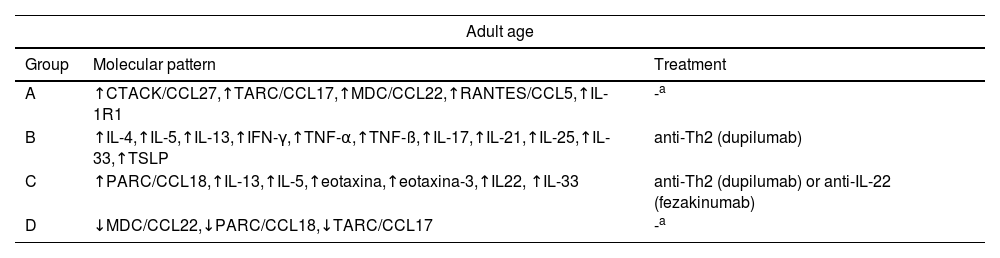
In this regard, Daphne et al.1 categorized 146 patients into 4 groups based on 143 serum biomarkers: group A, “cutaneous binding chemokines/dominant IL-1R1”; group B, “dominant Th1/Th2/Th17”; group C, “dominant Th2/Th22/PARC”; and group D, “inferior Th2/eosinophil”. Clinically, severity was significantly higher in group C, but no relevant differences were found regarding age, sex, age of onset, atopic comorbidities, or the length of stay. Groups B and C would probably respond better to dupilumab, and group C would potentially benefit from anti-IL-22 drugs. At the time of the study and with the available drugs, the authors could not make any therapeutic suggestions for groups A and D.
The previous study was replicated with a cohort of 240 children aged 0 to 17 years, resulting in 4 groups: group 1, “dominant Th2/retinol”; group 2, “dominant cutaneous binding”; group 3, “dominant Th1/Th2/Th17/IL-1”; and group 4, “Th1/IL-1/inferior eosinophil”. Only group 3 had its counterpart in adulthood: group B (Table 1). Although, clinically, the groups did not change by age, group 1 included more men, and group 2 showed a significantly higher severity and patients with food allergy. It has been suggested that groups 1 and 3 would have a greater response to anti-Th2 drugs, but at the time of the study, no therapeutic suggestions could be made for groups 2 and 4.2
Endotypes described in adults and children-adolescents, and suggested treatment.
| Adult age | ||
|---|---|---|
| Group | Molecular pattern | Treatment |
| A | ↑CTACK/CCL27,↑TARC/CCL17,↑MDC/CCL22,↑RANTES/CCL5,↑IL-1R1 | -a |
| B | ↑IL-4,↑IL-5,↑IL-13,↑IFN-γ,↑TNF-α,↑TNF-ß,↑IL-17,↑IL-21,↑IL-25,↑IL-33,↑TSLP | anti-Th2 (dupilumab) |
| C | ↑PARC/CCL18,↑IL-13,↑IL-5,↑eotaxina,↑eotaxina-3,↑IL22, ↑IL-33 | anti-Th2 (dupilumab) or anti-IL-22 (fezakinumab) |
| D | ↓MDC/CCL22,↓PARC/CCL18,↓TARC/CCL17 | -a |
| Childhood-adolescence | ||
|---|---|---|
| Group | Molecular pattern | Treatment |
| 1 | ↑RBP4,↑IL-4,↑IL-5,↑IL-13,↑TSLP,↑IL-23,↑IL-26 | anti-Th2 (dupilumab) |
| 2 | ↑Apelina,↑PARC/CCL18,↑TARC/CCL17,↑CTACK/CCL27,↓adiponectina, ↓MMP-8,↓TIMP1 | -a |
| 3 | ↑IL-2,↑IL-12,↑IFN-α,↑IFN-γ,↑TNF-α,↑TNF-ß,↑MIG/CXCL9, ↑ITAC/CXCL11,↑IL-4,↑IL-5,↑IL-13,↑eotaxina3/CCL26,↑TSLP, ↑MCP-4/CCL13,↑IL-23,↑IL26,↑MIP3a/CCL20,↑GM-CSF,↑IL-1a, ↑IL-1Ra,↑IL-1R1,↑IL-18BPa,↑IL-37,↑TNFR1,↑TNFR2, ↑TWEAK/TNFSF12,↑LIGHT/TNFSF14,↑sIL2Ra | anti-Th2 (dupilumab) |
| 4 | ↑RANTES/CCL5,↑PF4/CXCL4,↑marcador soluble de activación monocitarioCD14,↓MIG/CXCL9,↓ITAC/CXCL11,↓MIP1b/CCL2, ↓eotaxina-1/CCL11,↓eotaxina-3/CCL26,↓IL-1R1,↓IL-18BPa,↓TNFR1, ↓TNFR2,↓TWEAK/TNFSF12,↓elastasa,↓GCP2,↓sIL2Ra,↓CTACK | -a |
C-C motif MCP-4/CCL13, monocyte chemoattractant protein-4/chemokine ligand 13; C-C motif MDC/CCL22, macrophage-derived chemokine/chemokine ligand 22; C-C motif PARC/CCL18, pulmonary and activation-regulated chemokine/chemokine ligand 18; C-C motif TARC/CCL17, thymus and activation-regulated chemokine/chemokine ligand 17; CTACK/CCL27, chemokine (C-C motif)/ligand 27; CTACK, cutaneous T-cell attracting chemokine; C-X-C motif ITAC/CXCL11, IFN-inducible alpha T cells/a chemoattractant/chemokine (C-X-C motif) ligand 11; C-X-C motif MIG/CXCL9, gamma IFN-induced monokine/chemokine ligand 9; GM-CSF, granulocyte-macrophage colony-stimulating factor; LIGHT/TNSF14, tumor necrosis factor superfamily member 14; MIP, macrophage inflammatory proteins; MMP, matrix metalloproteinases; PF C-C motif RANTES/CCL5, platelet-derived factor regulated upon activation of normal T cells, compacted and secreted/chemokine ligand 5; RBP, retinol-binding protein; sIL2RA, mouse soluble interleukin-2 receptor alpha; TIMP, tissue inhibitors of metalloproteinases; TSLP, thymic stromal lymphopoietin; TWEAK/TNSF12, tumor necrosis factor-like weak inducer of apoptosis/tumor necrosis factor superfamily member 12.
At the time of the study, and with the available therapies, no therapeutic suggestions were made for adult age groups A and D or pediatric age groups 2 and 4. Data extracted from Bakker et al.1,2.
Additionally, some clinical trials have identified isolated predictive biomarkers. The trial with fezakinumab revealed that the greater expression of IL-22 in skin biopsies correlated with a greater reduction in severity indices.6 Similarly, the trial with tralokinumab revealed that the best response was associated with increased levels of serum DPP-4 and periostin.7
Biomarkers could improve screening, diagnosis, prognosis, monitoring of AD severity, and prediction of associated comorbidities, with the corresponding early approach and lower morbidity and mortality. However, none of these are validated or used in the routine clinical practice, which is why high-quality studies are needed to identify clinically relevant, accurate, and cost-effective biomarkers, preferably obtained by non-invasive methods such as tape stripping, blood, serum, or saliva.3–5The current clinical guidelines follow a single model without bearing in mind the uniqueness of each patient. Its counterpart is precision medicine, which advocates for biomarker based selected treatments. The mentioned authors have been able to identify which groups of patients would presumably respond better to anti-Th2 and anti-IL22; perhaps in the future, drugs will be developed vs the impaired pathways in the remaining groups. Due to limited health care resources and the high cost of available biologics and JAK inhibitors, this strategy could avoid the use of inappropriate drugs associated with impaired pathways.