Primary cutaneous cribriform carcinoma (PCCC) is a rare subtype of low-grade apocrine intraductal carcinoma with a characteristic histologic pattern, which consists of islets of epithelial cells and small cystic spaces that acquire a sieve-like appearance. Clinically, it presents as a firm nodule with no specific characteristics, usually located on the upper and lower extremities of middle-aged women.1
A 47-year-old woman visited our department with a lesion on the back that had appeared 9 months earlier. She had no relevant medical history and her general health status was good. Physical examination revealed a firm, grayish-brown nodule with a diameter of approximately 1cm in the region of the left scapula. A simple excision of the lesion was performed.
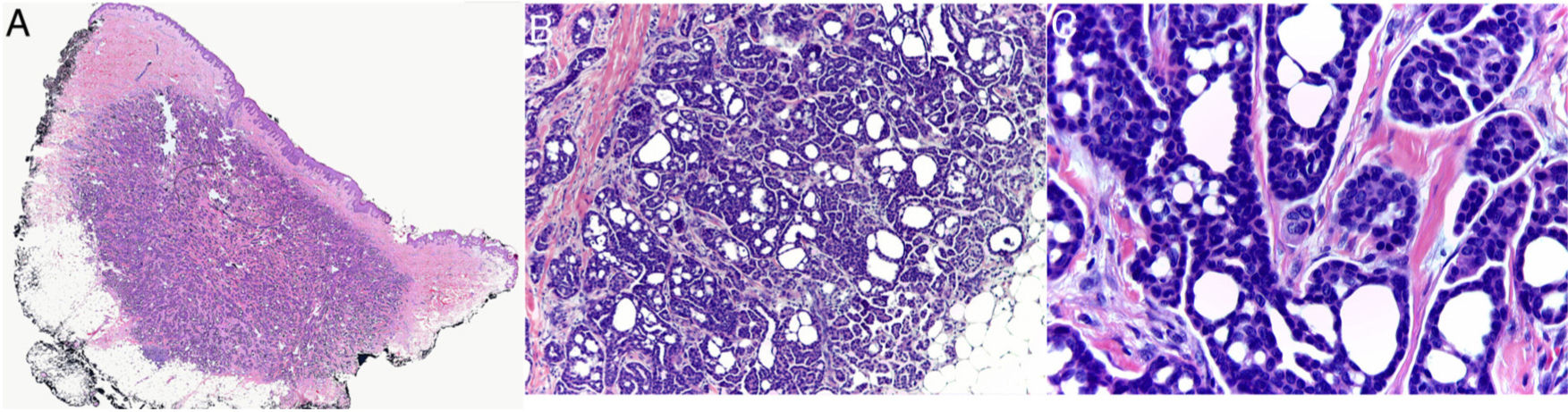
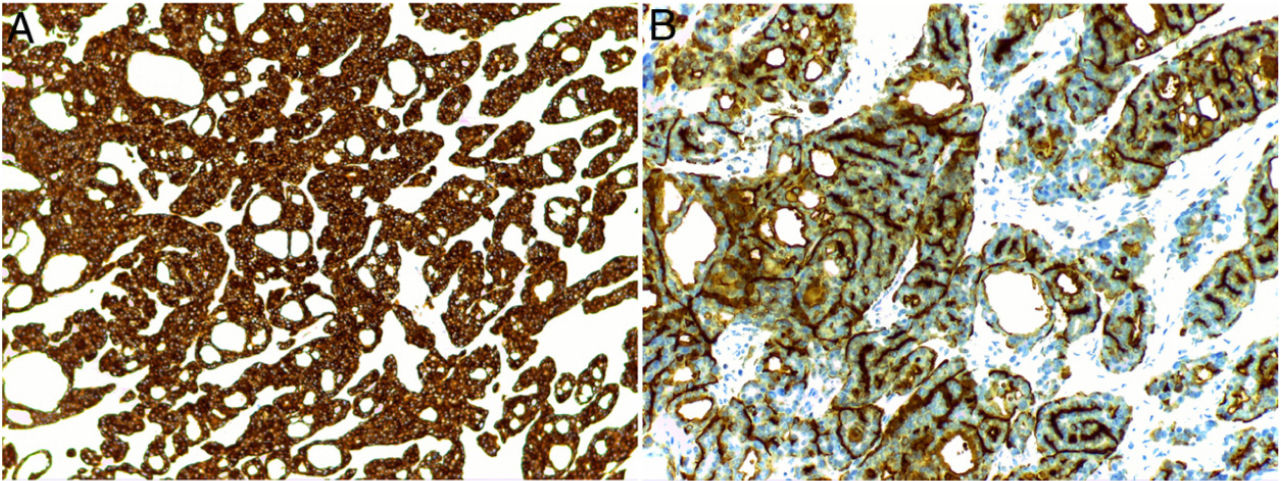
Histology revealed a partially circumscribed nonencapsulated dermal proliferation of epithelial cells. The lesion was spreading locally toward the subcutaneous cellular tissue and presented no connection with the overlying epidermis (Fig. 1A). It was composed of interconnected islets of epithelial cells that formed areas of ductal differentiation, acquiring a cribriform pattern throughout (Fig. 1B). The tumor cells showed scant cytoplasm and had large, basophilic, and moderately pleomorphic nuclei. The cystic spaces showed variable morphology, were empty, and showed no deposit in the basement membrane. Some of them showed intraluminal bridges and the formation of micropapillae (Fig. 1C). The stroma was collagenous and sparse, though more abundant in the center of the lesion. The periphery of the tumor showed nodular lymphoid aggregates. No perineural or intravascular invasion was identified. Mitotic figures were scant and tumor necrosis was not present. The proliferative index with Ki-67 was between 5% and 10%. Immunohistochemical analysis showed the tumor cells to be positive for CK7 (Fig. 2A) and the ductal structures were stained with CEA and EMA (Fig. 2B). However, they were negative for CK20, CDX2, GCDFP-15, S-100, estrogen receptor, progesterone receptor, mammaglobin, smooth-muscle actin, and p63.
(A) Panoramic image of the biopsy showing a partially circumscribed proliferation in the dermis that extends focally to the subcutaneous cellular tissue (hematoxylin–eosin, ×10). (B) The tumor is composed of multiple interconnected islets of basophilic epithelial cells that form small, round, empty cystic spaces, taking on a sieve-like pattern (hematoxylin–eosin, ×40). (C) The nuclei of the neoplastic cells are large and moderately pleomorphic. Some of the cystic spaces show the presence of intraluminal bridges and the formation of micropapillae (hematoxylin–eosin, ×400).
A histologic diagnosis of cribriform carcinoma was proposed and a complete study was performed to rule out primary visceral neoplasm. Mammogram, breast ultrasound, and PET/CT scans revealed no relevant findings and the diagnosis of PCCC was therefore confirmed. The patient presented no local recurrence or metastasis of the tumor after 17 months of follow-up.
PCCC is a rare histologic variant of cutaneous apocrine carcinoma, first described in 1998 by Requena et al.2 Its biologic behavior appears to be indolent, as cases of recurrence or metastasis of the tumor after complete resection have not been reported.1,3 Nevertheless, it is considered to be a low-grade malignant tumor because, histologically, it may present irregular edges, focal necrosis, or nuclear pleomorphism. The treatment of choice is therefore surgical resection with wide margins.1
The histologic differential diagnosis includes adenoid cystic carcinoma, a locally aggressive tumor with the ability to metastasize and which shows an infiltrating cribriform growth pattern and a tendency toward neurotropism. The presence of basophilic mucinous material in the cystic spaces, the lack of intraluminal bridges, and the presence of deposits of basal membrane material may help to differentiate this entity from PCCC.4,5 Moreover, the markers p63 and smooth-muscle actin are useful, as they show the presence of a layer of myoepithelial cells that is absent in PCCC.5 Other primary skin tumors that may present a cribriform pattern, generally focally, are basal cell carcinomas with an adenoid cystic pattern, syringomatous carcinoma, hidradenoma, and hidradenocarcinoma. These tumors also present other characteristic histologic traits that guide diagnosis, such as the presence of palisading cells on the periphery of the tumoral islets of basal cell carcinoma, the characteristic distal ductal differentiation of syringomatous carcinoma, or the solid islets with necrosis of hidradenocarcinoma.4
The principal differential diagnosis of PCCC, however, are the cutaneous metastases of visceral carcinomas from the prostate, colon, endometrium, salivary glands, thyroid, and breast. The absence of prior oncologic history and negativity for the immunohistochemical markers GCDFP-15, CK20, CDX2, mammaglobin, and hormone receptors (estrogen receptor/progesterone receptor) militate against the diagnosis of cutaneous metastasis. Nevertheless, imaging studies (CT and/or PET) should be performed to rule out an undetected visceral primary carcinoma before concluding a diagnosis of PCCC.1,3
In summary, PCCC is a rare tumor that must be considered as a potential diagnosis in the event of a cutaneous tumor with a cribriform pattern. Given its indolent biologic behavior, it must be differentiated from metastatic cribriform carcinoma and from adenoid cystic carcinoma. The histopathology and immunohistochemical characteristics supported by the clinical history and imaging studies are essential to reaching the correct diagnosis.
Conflicts of interestThe authors declare that they have no conflicts of interest.