Squamous cell carcinoma (SCC) of the nail unit is a very uncommon, slow-growing tumor with good prognosis and few reported cases of metastasis (2–3%).1 Its incidence peaks in individuals aged between 50 and 69 years, and the tumor predominantly affects men (ratio of 2:1).2
We conducted a descriptive retrospective study of all patients diagnosed with nail SCC in the Hospital Universitario Fundación Alcorcón, Madrid, Spain, and treated with Mohs micrographic surgery (MMS) between January 2006 and December 2019. All study variables were extracted from the database of the Anatomical Pathology Department of the center. The data were introduced into an anonymized database and analyzed with the SPSS® statistics package (version 20.9, SPSS Inc., Chicago, IL, United States). The study was approved by the Ethics Committee for Medical Research of the Hospital Universitario Fundación Alcorcón.
Over 14 years (2006–2019) we have diagnosed and treated 9 patients (11 tumors) with nail SCC using MMS (Table 1): 6 men (66%) and 3 women (33%), with a median age of 62 years (range, 40-81 years). In 45% of patients, the tumors were on the thumb (5/11), 36% on other fingers of the hand (4/11), and 18% on the great toe (2/11). Two patients were immunosuppressed (HIV infection and myeloproliferative neoplasm).
Clinical and demographic characteristics of the patients diagnosed with nail squamous cell carcinoma.
| Sex | Age, y | Site | Time until diagnosis, y | Histology | Immunosuppression | MMS stages | Surgical defect closure | |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | Great toe | 8 | SCC | No | 6 | Secondary intention |
| 2 | M | 76 | Little finger | 6 | BCC | Yes | Amputation | |
| 3 | M | 43 | Right thumb | Unknown | SCC | No | 4 | Secondary intention |
| Left thumb | SCC | 3 | Secondary intention | |||||
| Index finger | BCC | 2 | Secondary intention | |||||
| 4 | F | 40 | Thumb | 2 | BCC | Yes | 3 | Secondary intention |
| 5 | M | 81 | Thumb | 2 | SCC | No | 3 | Secondary intention→amputation |
| 6 | M | 67 | Thumb | 2 | SCC | No | 1 | Graft |
| 7 | M | 60 | Middle finger | 3 | SCC | No | 4 | Secondary intention |
| 8 | M | 62 | Ring finger | 2 | SCC | No | 2 | Secondary intention |
| 9 | F | 76 | Great toe | 2 | SCC | No | 2 | Secondary intention |
Abbreviations: BD, Bowen disease; F, female; M, male; SCC, squamous cell carcinoma.
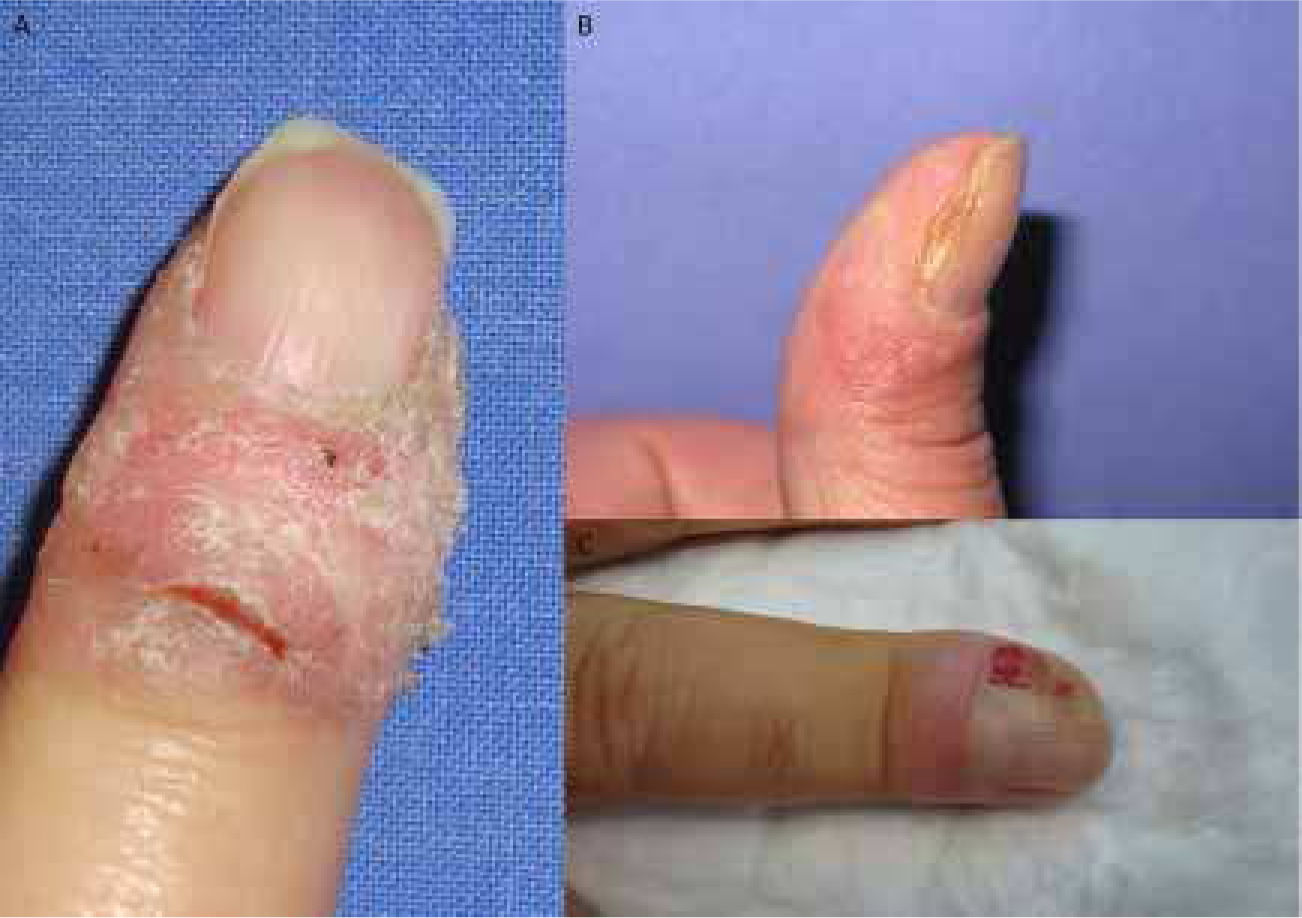
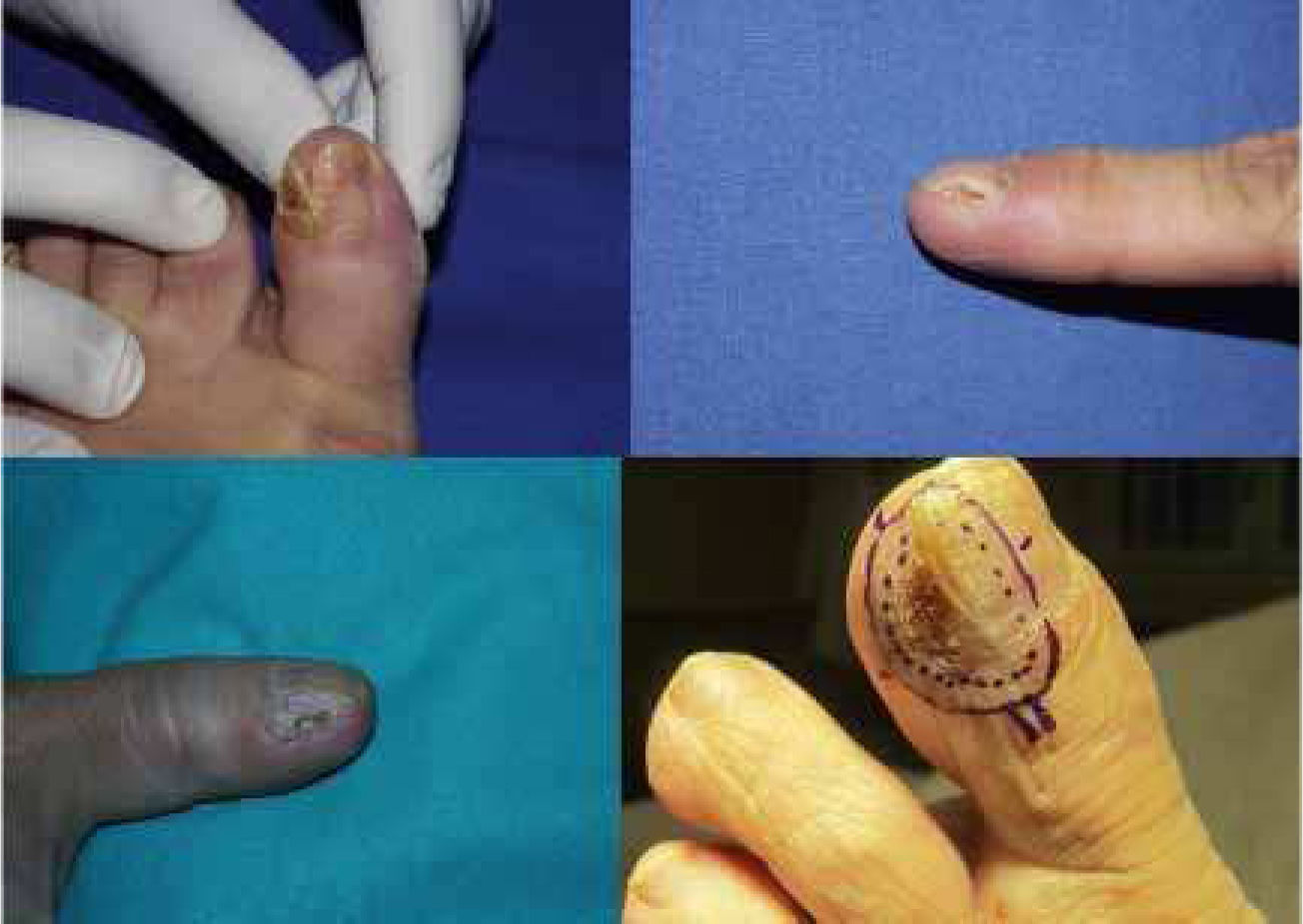
Clinically, the lesions presented as erythematous scaly plaques (Fig. 1A and B), keratotic nodules, or tissue resembling periungual granulation (Fig. 1C). In 50% of patients, there were nail bed abnormalities (onycholysis, hyperkeratosis, xanthonychia, or lateral wall destruction) (Fig. 2). In 4 out of 9 patients (44.4%) with large tumors or when pain was present, we performed a simple X-ray of the affected digit before surgery to rule out bone involvement.
All patients underwent MMS with a median of 3 stages (range, 1–6 stages). In 1 patient, MMS was not completed and after the first stage with positive margins, the patient opted for amputation of the phalanx. After achieving tumor-free margins, defect closure by secondary intention was performed in 9 of the 10 remaining tumors, while a skin graft was required in the other case. There were no complications after surgery. Histological study confirmed infiltrative SCC in 8 of the 11 tumors (72%) and in situ tumor in the remaining 3 (27%).
After a median follow-up of 9 years (108 months, range 9–254 months), 2 patients experienced local tumor recurrence after 2 and 10 years, respectively. The first patient presented in the clinic unexpectedly with pain and increased volume in the pulp of the affected finger. Magnetic resonance imaging revealed a heterogeneous mass in the dermis and subcutaneous tissue with destruction of the distal phalangeal bone. After histological confirmation of recurrence, the distal phalanx of the affected finger was amputated. The second patient, who was positive for HIV (stage C3), experienced recurrence after more than 10 years follow-up in the form of a poorly defined erythematous scaly plaque around the nail. Simple X-ray ruled out bone involvement. However, after histological confirmation of recurrence, the patient refused any further surgical intervention and is in close clinical follow-up. To date, no patient has died or experienced lymph node or distant metastasis.
Nail SCC is a very uncommon nail tumor. It is located mainly on the thumb and index finger.1,2 The clinical presentation is varied and not very specific, with the tumor often diagnosed as a benign process (periungual warts, paronychia, chronic eczema, and onychomycosis) thus delaying diagnosis.3–5 In our series, 63.6% of the tumors (7/11) were located on the thumb and index finger, with a median duration from lesion onset until histopathological diagnosis of 2 years (range, 2–8 years). In general, nail SCC does not follow an aggressive course compared with SCC at other anatomical sites such as the lip or pinna.6 In 30–40% of cases, bone involvement is detected on performing an imaging test.3,4,7 However, diagnosis of bone involvement is not always reliable and in many patients, it may correspond to a false positive due to inflammatory reaction or compression exercised by the tumor.7,8 In our series, we performed preoperative simple X-ray in 44.4% of the patients, and ruled out bone involvement in all cases. Most authors agree on the need for a preoperative imaging test for all patients diagnosed with nail SCC, regardless of tumor size or symptoms. This preoperative screening can be performed with simple X-ray, an accessible and reliable method for evaluating bone involvement prior to surgery. However, the specificity of this technique is not high, above all in tumors of exophytic growth, where false positives may be frequent. Therefore, in cases of doubt, magnetic resonance imaging is the most specific test for evaluating soft tissue and bone involvement.
Among the trigger factors described (radiation, arsenic, pesticides, congenital dyskeratosis), infection with oncogenic human papillomavirus (HPV) infection plays a primary role. HPV has been demonstrated to be responsible for nail SCC in up to 60% of cases, with serotype 16 being the most frequent (75%).9 Other serotypes identified include serotypes 2, 6, 11, 18, 26, 31, 34, 35, 56, 58, and 73. Up to a third of patients with nail SCC have a history of genital infection with HPV.
Photodynamic therapy, CO2 laser therapy, topical treatment with 5% 5-fluorouracil or 5% imiquimod, scraping, and electrochemotherapy are effective treatments for nail SCC. However, surgery is the treatment of choice, above all taking into account the possibility of local invasion and bone involvement. Although MMS is a challenge given the anatomical and histologic features of the nail unit, it is considered the most effective surgical technique for treatment of nail SCC, with cure rates of up to 96% and recurrence rates of between 0 and 22% compared with rates of 28.5%–56% reported for conventional nail surgery.4,7,10 MMS permits assessment of periosteal invasion in the deep part of the tumor and it can reliably distinguish bone invasion from inflammation or compression. In our series, after a median follow-up of 9 years, local recurrence was reported in 18.1% (2/11) of the tumors. A higher rate of local recurrences has been reported with increased number of stages required to achieve disease-free margins, and in HPV-positive tumors.
In conclusion, nail SCC is an uncommon malignant neoplasm. Diagnosis is often delayed with the lesion being confused with benign nail disease. MMS is the technique of choice for treatment, with a low rate of local recurrence while achieving good functionality and quality of life.
Conflicts of interestThe authors declare that they have no conflicts of interest.