Numerous surgical and nonsurgical modalities are available to treat basal cell carcinoma (BCC), but their true effectiveness and safety is unknown. This article summarizes the evidence presented in a recent Cochrane review and aims to facilitate the interpretation of the review's findings for the Spanish and Latin American scientific communities. Much of the evidence the reviewers found came from single studies, preventing meta-analysis. Conventional surgical excision continues to be the most effective treatment for low-risk BCC. Most studies had small sample sizes, and some had problems with blinding, limitations which will have affected the assessment of subjective outcomes, such as pain and cosmetic results. The authors identified a lack of standardization in relation to recurrences and cosmetic outcomes that threatens not only the internal validity of the studies but also their external validity and reproducibility.
Existen numerosas modalidades de tratamiento para el manejo de los carcinomas basocelulares (CBC), pero se desconoce la real eficacia y seguridad entre las alternativas quirúrgicas y no quirúrgicas disponibles. Este artículo resume la evidencia encontrada en la reciente revisión Cochrane de Thomson et al. y facilita la interpretación de sus resultados entre la comunidad científica iberolatinoamericana. La gran mayoría de la evidencia evaluada proviene de estudios individuales que impidieron la realización de una revisión sistemática cuantitativa. La escisión quirúrgica convencional continúa siendo la terapia más eficaz para el tratamiento de los CBC de bajo riesgo. La mayoría de los estudios incluyeron tamaños de muestra pequeños y algunos tuvieron problemas con el cegamiento, lo que influiría en resultados subjetivos, tales como el dolor o la cosmesis. Existe una falta de estandarización con relación a los desenlaces de recurrencia y de resultados cosméticos, lo que en conjunto afecta no solo la validez interna sino también la validez externa y la reproducibilidad de los estudios.
Basal cell carcinoma (BCC) is a slow-growing, locally invasive tumor. It is the most common skin cancer in humans.1
The true incidence of BCC is unknown. While these skin tumors are very common, it is estimated that some 30% to 50% are not reported, either because they are removed without prior confirmation by biopsy or because cancer registries in most countries are not required to report them.1,2 Although comparison of national incidence rates is often limited by differences in standardization methods and cancer registry processes, the highest rates have been reported in Queensland, Australia (1269 cases per 100000 person-years for women and 1813 cases per 100000 person-years for men)1,3 and California, the United States (1069 cases per 100000 person-years for men).4 The highest rate reported in mainland Europe has been in the Netherlands, with 164.7 cases per 100000 person-years.1,3 Higher rates have been linked to low geographic latitude. Increases of 2% to 5% in the incidence of BCC have been observed worldwide, with the exception of Australia, where cases have stabilized.1,5 Epidemiologically, it is worth noting that in Europe, BCC has a higher incidence in women and young people, possibly in relation to the use of tanning booths and a greater tendency among women than men to seek medical care.1,3
The main risk factors for BCC are older age, male sex, fair skin, low tanning ability, intermittent intense exposure to UV light during childhood, and cutaneous signs of actinic damage.1,3
The clinical and morphologic features of BCC are variable, with more than 26 subtypes described.1,6 The main clinical subtypes are nodular, superficial, ulcerated (rodent ulcers), morpheaform (sclerodermiform), fibroepithelial (fibroepithelioma of Pinkus), and advanced (invasive). Histologic subtypes also vary and comprise nodular, superficial, morpheaform, micronodular, macronodular, infiltrative, pigmented, and basosquamous (metatypical) patterns.1,7 The main histologic subtypes are nodular and superficial BCC, and the most common location is the head and neck area.1,8
Although BCCs are usually slow growing and have a very low metastatic potential (0.0028%–0.55%), left untreated, they can cause significant tissue destruction, particularly when located on the face, and they can even invade the bone and deeper structures.1,9 Clinical course is largely unpredictable, with some tumors remaining small for many years, others showing regression, and others growing rapidly and invading large areas of tissue.1,10 BCCs have also been classified into high- and low-risk subtypes. High-risk types include tumors with morpheaform, infiltrative, and micronodular patterns, perineural or perivascular invasion, or a diameter greater than 5cm, as well as recurrent tumors, tumors located in the center of the face or close to vital organs (periocular and periauricular tumors), and tumors in immunosuppressed patients. Low-risk types include superficial and nodular BCCs in low-risk areas.1,6
BCC poses a considerable burden on health care systems due to its high and growing incidence and associated morbidity. Patients are also more likely to develop other BCCs and skin tumors linked to UV light exposure.1,3 Accordingly, associated disability-adjusted life years and medical costs have increased significantly in recent decades.1,3
Numerous surgical and nonsurgical modalities are available to treat BCC, but their true effectiveness and safety is unknown.1 The original Spanish version of this article is part of a series to be published in 5 issues of Actas Dermo-Sifiliográficos over the course of a year. Its purpose is to summarize the evidence presented by Thomson et al.1 in the recent Cochrane review and facilitate the interpretation of the review's findings for the Spanish and Latin American scientific communities.
MethodsThe authors of the original review used a previously published protocol.11
Search StrategiesThomson et al.1 searched the Cochrane Skin Group Specialized Register, the Cochrane Central Register of Controlled Trials, MEDLINE and Embase via Ovid, CINAHL via EBSCO, LILACS, and 5 clinical trial registers. They also checked the references of the studies included, and where necessary contacted authors for additional information. The date of the last search was November, 2019. Three authors independently reviewed the titles, abstracts, and full texts, and discrepancies were resolved by a fourth author.
Inclusion CriteriaThe authors included randomized controlled trials (RCTs) evaluating surgical and nonsurgical interventions for the treatment of any type of BCC in immunocompetent patients with a biopsy-confirmed diagnosis. They excluded studies of patients with persistent or recurrent tumors and syndromes with a high risk of BCC (e.g., Gorlin syndrome).
ComparatorsComparators were placebo, active treatment, other treatments, or no treatment.
Outcome MeasuresThe primary outcome measures were 1) clinical recurrence at 3–5 years or any time if no information was available for this period and 2) cosmetic outcomes rated as good or excellent by the patient or an observer (investigator).
Secondary outcome measures were 1) pain during and after treatment, 2) treatment failure in the first 6 months, and 3) adverse events (AEs).
Bias and Quality of EvidenceBias was assessed using the Cochrane risk of bias tool. Risk was assessed separately by 2 authors, and discrepancies were resolved by discussion with a third.
Quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.
ResultsThe main interventions used to treat BCC were surgical excision (SE), Mohs micrographic surgery (MMS), photodynamic therapy with methylaminolevulinate (MAL-PDT), photodynamic therapy with aminolevulinic acid (ALA-PDT), and topical imiquimod. Additional treatments were radiotherapy, ablative fractional laser-assisted MAL-PDT, cryosurgery, fluorouracil, intralesional interferon, pulsed dye laser, and ingenol mebutate.
The authors included 52 RCTs reported in 75 articles and involving 6690 patients and 7241 lesions. Trial duration ranged from 6 weeks to 10 years (mean, 13 months). Most trials analyzed low-risk BCCs (superficial and nodular subtypes) only. Approximately 22 trials involving imiquimod and PDT were funded by the pharmaceutical industry. All trials were prospective and had a parallel-group design. The median age of participants in the trials that reported age was 64.9 years (range, 20–95 years). The male to female ratio in those that reported patient sex was 1.48:1.
Bias AssessmentRandom Sequence Generation and Allocation ConcealmentTwenty-nine trials had a low risk of bias for random sequence generation, while 22 had an unclear risk. In the case of allocation concealment, 23 trials had a low risk of bias and 28 had an unclear risk. Just 1 trial had a high risk of bias for random sequence generation and allocation concealment.
BlindingFourteen trials had a low risk of bias for participant blinding, while 38 trials had an unclear risk.
In the case of outcome assessment blinding, 19 trials had a low risk of bias and 33 had an unclear risk.
Incomplete Outcome DataThirty-two trials had a low risk of bias for incomplete outcome data, while 19 had an unclear risk. One trial had a high risk of bias in this area due to a large between-group difference in the number of participants who withdrew.
Selective ReportingJust 11 trials preregistered the study protocol, and of these, 6 had a low risk of bias for selective reporting of outcomes. Forty-four trials had an unclear risk of bias in this area, while 2 had a high risk.
Eight trials were described as pilot studies. Twenty trials compared one nonsurgical treatment with another, while 14 trials compared a nonsurgical treatment with placebo. Surgical treatment was used as a comparator in 18 trials. It was compared with a nonsurgical treatment in 10 trials, another surgical treatment in 5, and placebo in 3.
Effect of Interventions on the 7 Main Comparisons (See the Original Review1 for Other Comparisons and Doses)MMS vs. SEJust 1 trial, involving 374 participants and 408 primary BCCs with a high-risk histologic subtype located in the H zone of the face, compared MMS and SE.
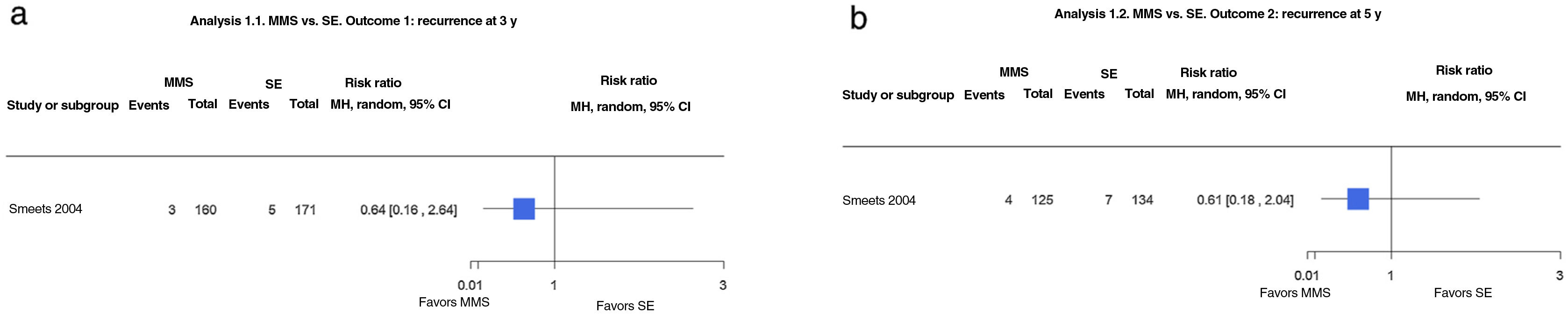
The authors detected slightly fewer recurrences for MMS compared with SE at both 3 years (1.9% vs. 2.9%; risk ratio [RR], 0.64; 95% CI, 0.16–2.64; low-certainty evidence) and 5 years (3.2% vs. 5.2%; RR, 0.61; 95% CI, 0.18–2.04; low-certainty evidence) (Fig. 1A, B).
(1.1 and 1.2) Mohs micrographic surgery (MMS) vs. surgical excision (SE). Outcome: recurrence at 3 and 5 years (analyses 1.1 and 1.2 in the original review1). Interpretation: moderate-certainty evidence. Just 1 study analyzed recurrence at 3 and 5 years. Evidence in favor of SE for reduction in risk of recurrence at 3 and 5 years, but low certainty due to imprecision (wide confidence interval). MH indicates Mantel–Haenszel.
The evidence supporting the effects of MMS and SE on cosmetic outcomes and adverse events (AEs) is uncertain as these were not analyzed in the original trial.
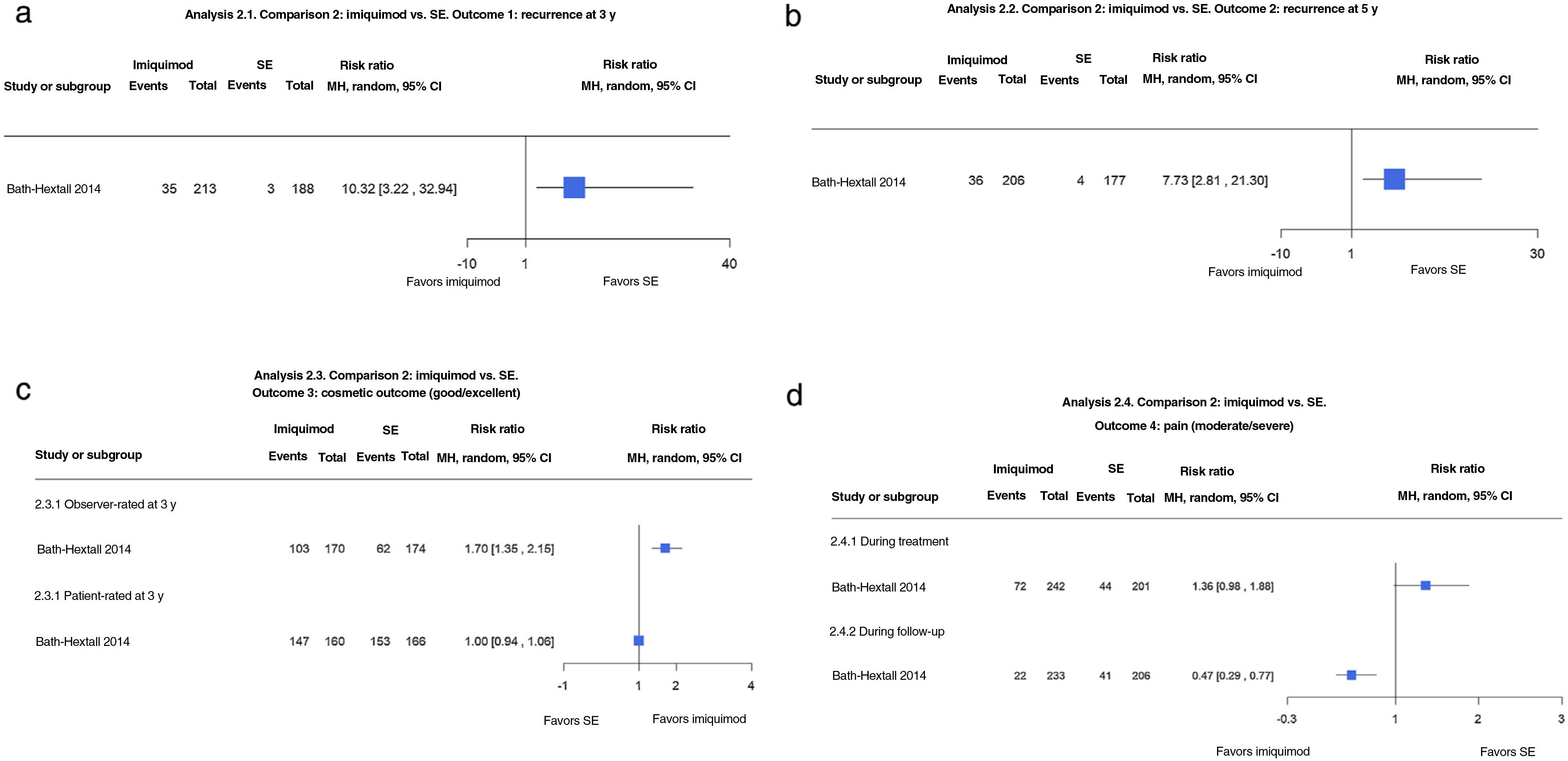
Imiquimod vs. SEOne noninferiority trial compared imiquimod with SE in 501 patients with nodular and superficial BCCs in low-risk areas. The authors of the Cochrane review concluded that imiquimod probably results in higher recurrence rates than SE at both 3 years (16.4% vs. 1.6%; RR, 10.30; 95% CI, 3.22–32.94; moderate-certainty evidence) and 5 years (17.5% vs. 2.3%; RR, 7.73; 95% CI, 2.81–21.30; moderate-certainty evidence) (Fig. 2A, B).
(2.1 and 2.2) Imiquimod vs. surgical excision (SE). Outcome: recurrence at 3 and 5 years, respectively (analyses 2.1 and 2.2 in the original review1). Interpretation: moderate-certainty evidence. Just 1 study analyzed recurrence at 3 and 5 years. Evidence in favor of SE for reduction in risk of recurrence at 3 and 5 years with a wide confidence interval, indicating imprecision. (2.3) Imiquimod vs. SE. Outcome: good/excellent cosmetic outcome (analysis 2.3 in the original review1). Interpretation: low-certaintly evidence in favor of imiquimod. Just 1 study with a risk of blinding bias. (2.4) Imiquimod vs. SE. Outcome: moderate/severe pain (analysis 2.4 in the original review1). Interpretation: low-certainty evidence in favor of imiquimod during follow-up. Just 1 study with a high risk of blinding bias and dropout bias in the evaluation of pain during treatment in the SE group. MH indicates Mantel–Haenszel.
No differences were observed between the 2 treatments for patient-rated cosmetic outcomes at 6 months or 3 years, with 91.6% of patients in the imiquimod group and 92.2% of those in the SE group reporting good or excellent outcomes (RR, 1.00; 95% CI, 0.94–1.06; low-certainty evidence). Observer-rated outcomes, by contrast, were better for imiquimod, with 60.6% deemed good or excellent compared with 35.6% for SE (RR, 1.70; 95% CI, 1.35–2.15; low-certainty evidence) (Fig. 2C).
A higher proportion of patients treated with imiquimod reported pain during treatment (30% vs. 22% for those who underwent SE; RR, 1.36; 95% CI, 0.98–1.88; low-certainty evidence). The opposite, however, was observed, at 16 weeks, with just 9% of patients in the imiquimod group reporting pain compared with 20% in the SE group (RR, 0.47; 95% CI, 0.29–0.77; low-certainty evidence) (Fig. 2D).
Mild to moderate AEs were slightly more common in the imiquimod group (94% vs. 88% in the SE group). Itching and weeping were considerably more common in patients treated with imiquimod compared with SE (85% vs. 56% and 64% vs. 35%, respectively). In the only trial to analyze AEs, 5 patients in the imiquimod group (vs. none in the SE group) dropped out due to treatment-related AEs. Thirty-eight patients treated with imiquimod (15%) required a dose reduction due to AEs.
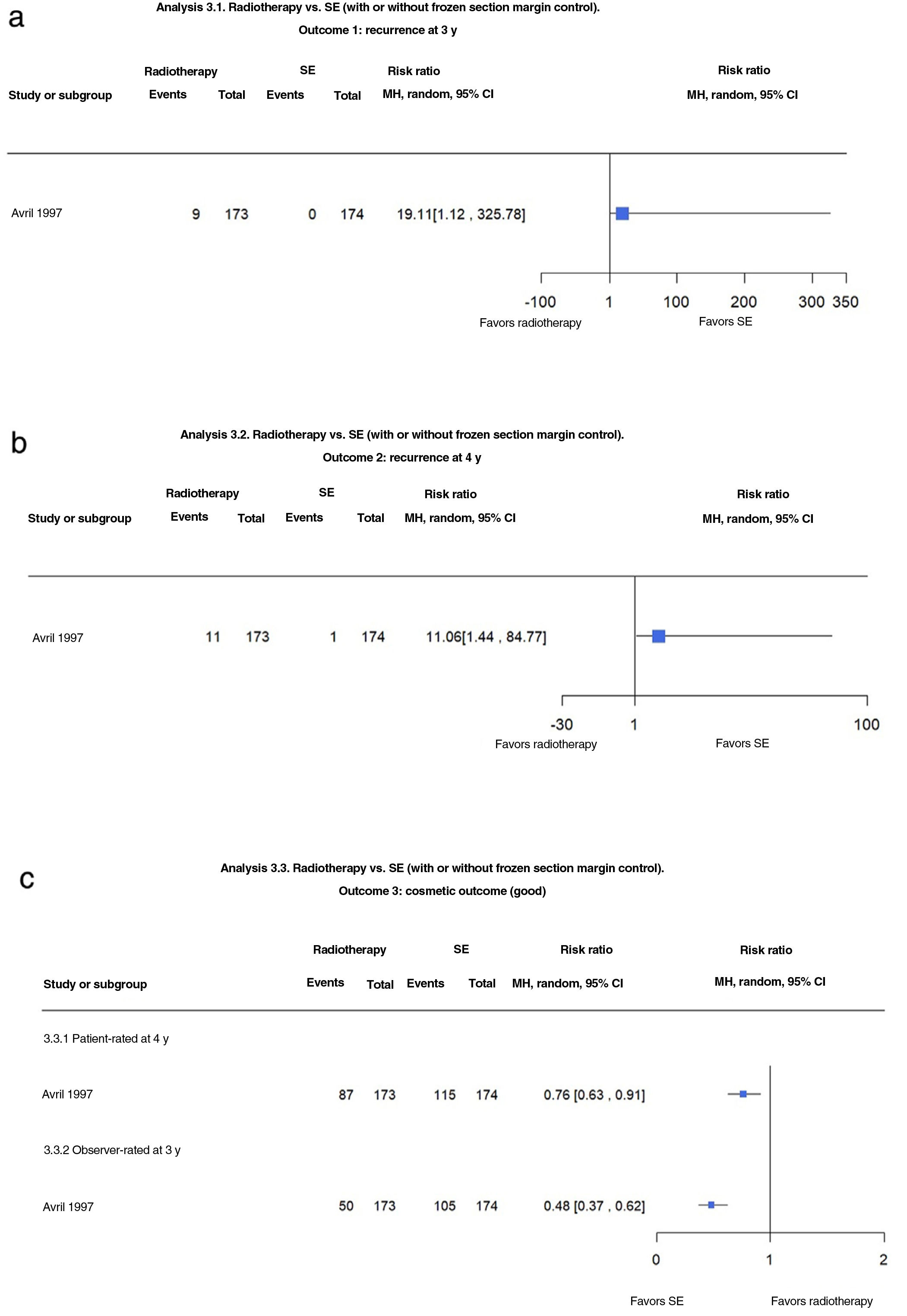
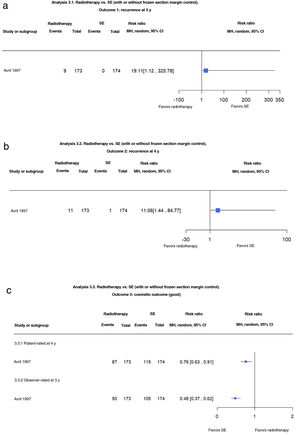
Radiotherapy vs. SE With and Without Frozen Section Margin ControlJust 1 trial compared radiotherapy and SE with or without frozen section margin control in 374 patients with facial BCC (low- and high-risk histologic subtypes) measuring less than 4cm. Radiotherapy was associated with a recurrence rate of 5.2% at 3 years compared with 0% for SE (RR, 19.11; 95% CI, 1.12–325.78; low-certainty evidence). Recurrences were also more common with radiotherapy at 4 years (6.4% vs. 0.6% for SE) (RR, 11.06; 95% CI, 1.44–84.77; low-certainty evidence) (Fig. 3A, B).
(3.1 and 3.2) Radiotherapy vs. surgical excision (SE) with and without frozen section margin control. Outcome: recurrence at 3 and 4 years (analyses 3.1 and 3.2 in the original review1). Interpretation: low-certainty evidence in favor of SE based on just 1 study with a risk of bias due to indirect evidence. Imprecision (wide confidence intervals) in the evaluation of the effect on recurrence at 3 and 4 years. (3.3) Radiotherapy vs. SE with and without frozen section margin control. Outcome: patient- and observer-rated cosmetic outcome (analysis 3.3 in the original review1). Interpretation: moderate-certainty evidence in favor of SE from just 1 study with a risk of blinding bias. Narrow confidence intervals for estimated effect on patient- and observer-rated cosmetic outcomes. MH indicates Mantel–Haenszel.
Cosmetic outcomes assessed on a clinical scale of poor, fair, and good at 4 years were more likely to be rated unfavorably in the radiotherapy group compared with the SE group (RR, 0.76; 95% CI 0.63–0.91; moderate-certainty evidence) (Fig. 3C).
Similar results were observed for observer-rated outcomes (RR 0.48; 95% CI, 0.37–0.62; moderate-certainty evidence).
Dyspigmentation and telangiectasia at 4 years were more common in patients treated with radiotherapy (>65%), as were cutaneous radiodystrophy (41.5%), necrosis (25%), and scar deformations and constrictions (5%).
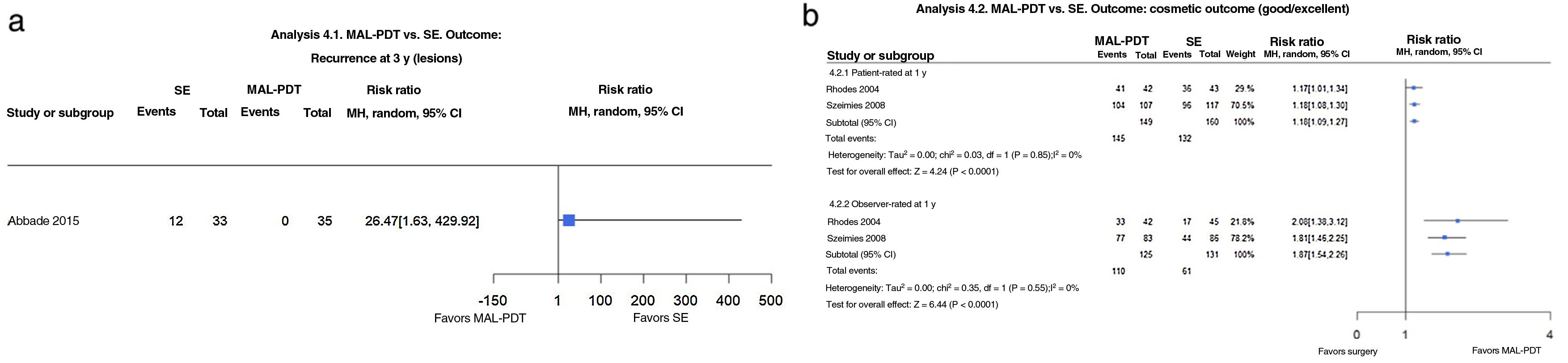
MAL-PDT vs. SESE was compared with MAL-PDT in 3 trials12–14 and ALA-PDT in 1.15 The trial by Rhodes et al.12 included 103 patients with a total of 118 nodular facial BCCs and follow-up of 5 years. The authors, however, performed a per-protocol analysis, providing insufficient data for Thomson et al.1 to determine the denominators for recurrence rates. Compared with SE, MAL-PDT appears to be associated with better patient- and observer-rated cosmetic outcomes (RR, 1.17; 95% CI, 1.01–1.34 and RR, 2.08; 95% CI, 1.38–3.12, respectively), but more pain and a burning sensation of the skin (13.4% vs. 6.1%; RR, 2.20; 95% CI, 0.60–8.03). Overall, AEs (burning sensation of the skin, pain in the skin, and erythema) were more probable in patients treated with MAL-PDT (52% vs. 29%) (P=.03, Fisher exact test). Three patients in the SE group developed a severe skin infection. Abbade et al.14 found that MAL-PDT was more likely to result in recurrence than SE (36.36% vs. 0%; RR, 26.47; 95% CI, 1.63–429.92) in a study of 57 patients with nodular BCC in the head and neck area (68 lesions) and a follow-up of more than 3 years. The risk of early treatment failure was also higher for PDT (RR, 11.65; 95% CI, 0.67–202.74)1,14 (Fig. 4A).
(4.1) Photodynamic therapy with methylaminolevulinate (MAL-PDT) vs. surgical excision (SE). Outcome: recurrence at 3 years (analysis 10.1 in the original review1). Interpretation: low-certainty evidence from just 1 study with a high risk of bias and imprecision in the evaluation on the estimated effect of recurrence at 3 years (wide confidence interval [CI]). (4.2) MAL-PDT vs. SE. Outcome: good/excellent cosmetic outcome (analysis 10.2 in the original review1). Interpretation: moderate-certainty evidence in favor of MAL-PDT from a single study with a high risk of blinding bias, with narrow CIs for patient-rated outcomes and wide CIs (imprecision) for observer-rated outcomes. MH indicates Mantel–Haenszel.
In a noninferiority trial, Szeimies et al.13 compared MAL-PDT and SE in 196 patients with 246 superficial BCCs located anywhere except the facial H zone; they were followed for 1 year.13 The inferiority margin was a difference of 15% in the percent reduction in lesion count 3 months after the last treatment. Both patient- and observer-rated cosmetic outcomes were more favorable for MAL-PDT, with respective RRs of 1.18 (95% CI, 1.08–1.30) and 1.81 (95% CI, 1.46–2.25) (Fig. 4B).
Szeimies et al.13 reported pain during and after treatment, attributing it to photosensitivity reactions that are expected to occur with PDT (e.g., skin discomfort, burning sensation, stinging, and erythema). AEs were reported for 37% of patients treated with MAL-PDT vs. 14% of those who underwent SE. One patient in the SE group developed a severe skin infection.
In a study of 149 patients with 171 nodular BCCs and a follow-up of 5 years, Mosterd et al.15 observed a 3-year recurrence rate of 24.7% for those treated with ALA-PDT and 2.3% for those treated with SE (RR, 10.87; 95% CI, 2.63–44.95; moderate-certainty evidence). ALA-PDT also appeared to result in more recurrences at 5 years (27.1% vs. 2.3%; RR, 11.91; 95% CI, 2.90–48.95; moderate-certainty evidence). SE appeared to improve the risk of early treatment failure compared with ALA-PDT (7.2% vs. 2.3%; RR, 3.18; 95% CI, 0.66–15.32; low-certainty evidence). One severe infection was also reported following SE in this trial.
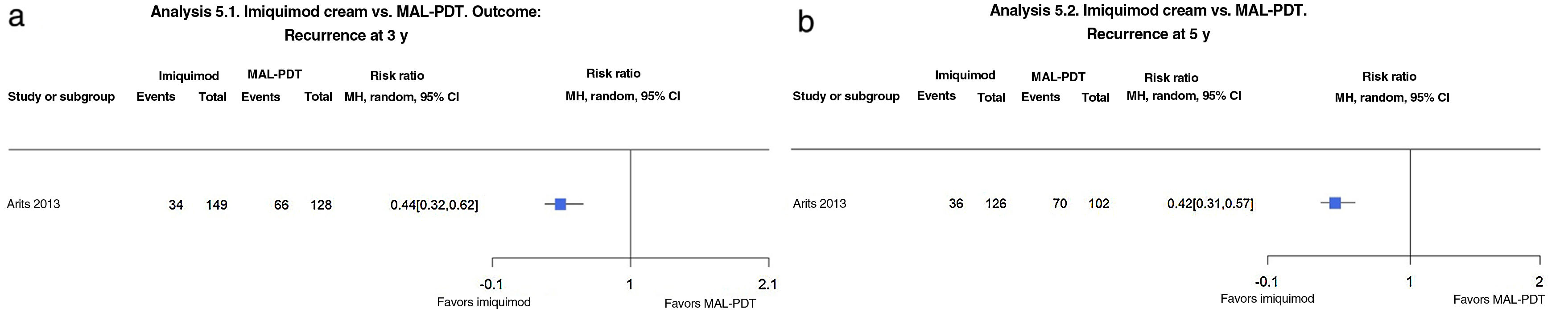
Imiquimod vs. MAL-PDTArits et al.1,16 conducted a noninferiority trial with a follow-up time of 5 years comparing 5-fluorouracil (5-FU) cream and imiquimod cream with PDT-MAL in 601 patients with a single high-risk BCC located anywhere except the face or scalp. Imiquimod compared with MAL-PDT probably reduces the risk of recurrence at both 3 years (22.8% vs. 51.6%; RR, 0.44; 95% CI, 0.32–0.62; moderate-certainty evidence) and 5 years (28.6% vs. 68.6%; RR, 0.42; 95% CI, 0.31–0.57; moderate-certainty evidence) (Fig. 5A, B).
(5.1 and 5.2) Imiquimod vs. photodynamic therapy with methylaminolevulinate (MAL-PDT). Outcome: recurrence at 3 and 5 years, respectively (analyses 18.1 and 18.2 in the original review1). Interpretation: moderate evidence from just 1 study showing favorable results for imiquimod in the reduction of recurrence at 3 and 5 years. MH indicates Mantel–Haenszel.
No significant differences were observed for good or excellent cosmetic outcomes at 1 year (RR, 0.98; 95% CI, 0.84–1.16; moderate-certainty evidence).1,16
Thomson et al.1 showed that imiquimod probably reduces the risk of severe pain compared with MAL-PDT (RR, 0.60; 95% CI, 0.41–0.87; moderate-certainty evidence).
Nonsignificant differences were observed for treatment failures at 3 months (10.1% for imiquimod vs. 15.8% for MAL-PDT; RR, 0.64; 95% CI, 0.37–1.09; moderate-certainty evidence).
Patients treated with imiquimod had higher rates of edema, erosion, crust formation, and itching; 4.8% developed a serious AE (8 cases of flu-like symptoms and 1 local wound infection treated in an outpatient setting).
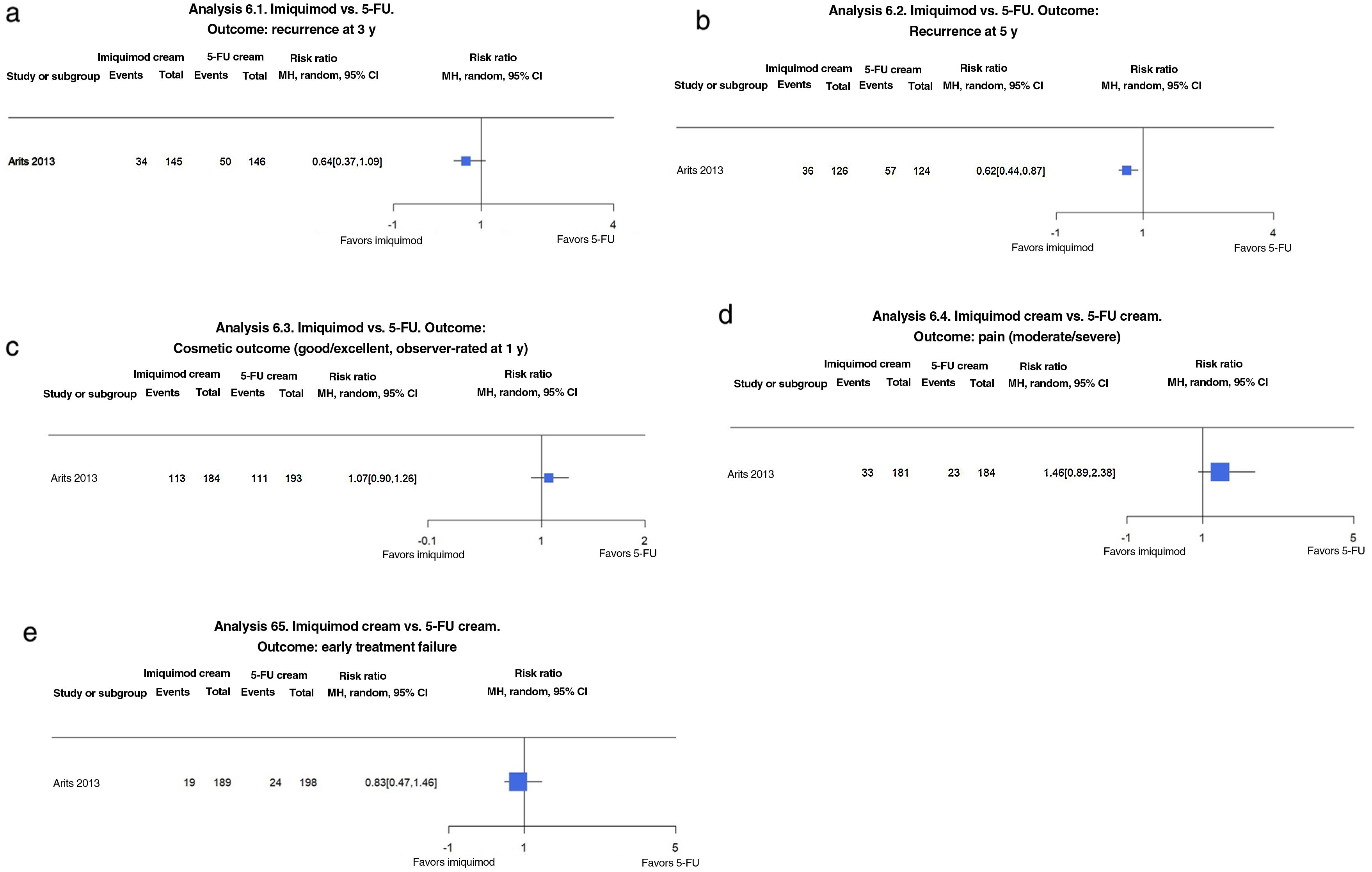
Imiquimod vs. Topical 5-FUIn the trial by Arits et al.16 imiquimod seemed to reduce the risk of recurrence at 3 years compared with topical 5-FU (23.4% vs. 34.2%; RR, 0.68; 95% CI, 0.47–0.99; moderate-certainty evidence). A similar finding was made for recurrence at 5 years (28.6% vs. 46%; RR, 0.62; 95% CI, 0.44–0.87; moderate-certainty evidence)1,16 (Fig. 6A, B).
(6.1 and 6.2) Imiquimod vs. 5-fluorouracil (5-FU). Outcome: recurrence at 3 and 5 years, respectively (analyses 23.1 and 23.2 in the original review1). Interpretation: moderate evidence from just 1 study showing favorable results for imiquimod in the reduction of recurrence at 3 and 5 years. Inconclusive findings. (6.3) Imiquimod vs. 5-FU. Outcome: good/excellent observer-rated cosmetic outcome at 1 year (analysis 23.3 in the original review1). Interpretation: Moderate evidence with just 1 study in favor of imiquimod for cosmetic outcome. Inconclusive findings. (6.4) Imiquimod vs. 5-FU. Outcome: moderate/severe pain (analysis 2.4 in the original review1). Interpretation: Moderate evidence with just 1 study in favor of 5-FU for cosmetic outcome. Inconclusive findings. (6.5) Imiquimod vs. 5-FU. Outcome: early treatment failure (analysis 23.5 in the original review1). Interpretation: low evidence with just 1 study in favor of imiquimod for early treatment failure. Inconclusive findings. MH indicates Mantel–Haenszel.
No significant differences were observed for cosmetic outcomes rated as good or excellent at 1 year (61.4% for imiquimod vs. 57.5% for 5-FU; RR, 1.07; 95% CI, 0.90–1.26; moderate-certainty evidence) (Fig. 6C).
Imiquimod was associated with a slightly higher risk of pain (18.2 vs. 12.5%; RR, 1.46; 95% CI, 0.89–2.34; moderate-certainty evidence) (Fig. 6D).
No significant differences were observed for early treatment failures (10% for imiquimod vs. 12.1% for 5-FU; RR, 0.83; 95% CI, 0.47–1.46; low-certainty evidence) (Fig. 6E).
Patients treated with imiquimod and 5-FU experienced higher rates of edema, erosion, crusting, and pruritus than those treated with MAL-PDT. Two patients in the 5-FU group developed a local wound infection managed in an outpatient setting. This treatment was also associated with erysipelas of the lower limb in one patient and a leg ulcer in another.
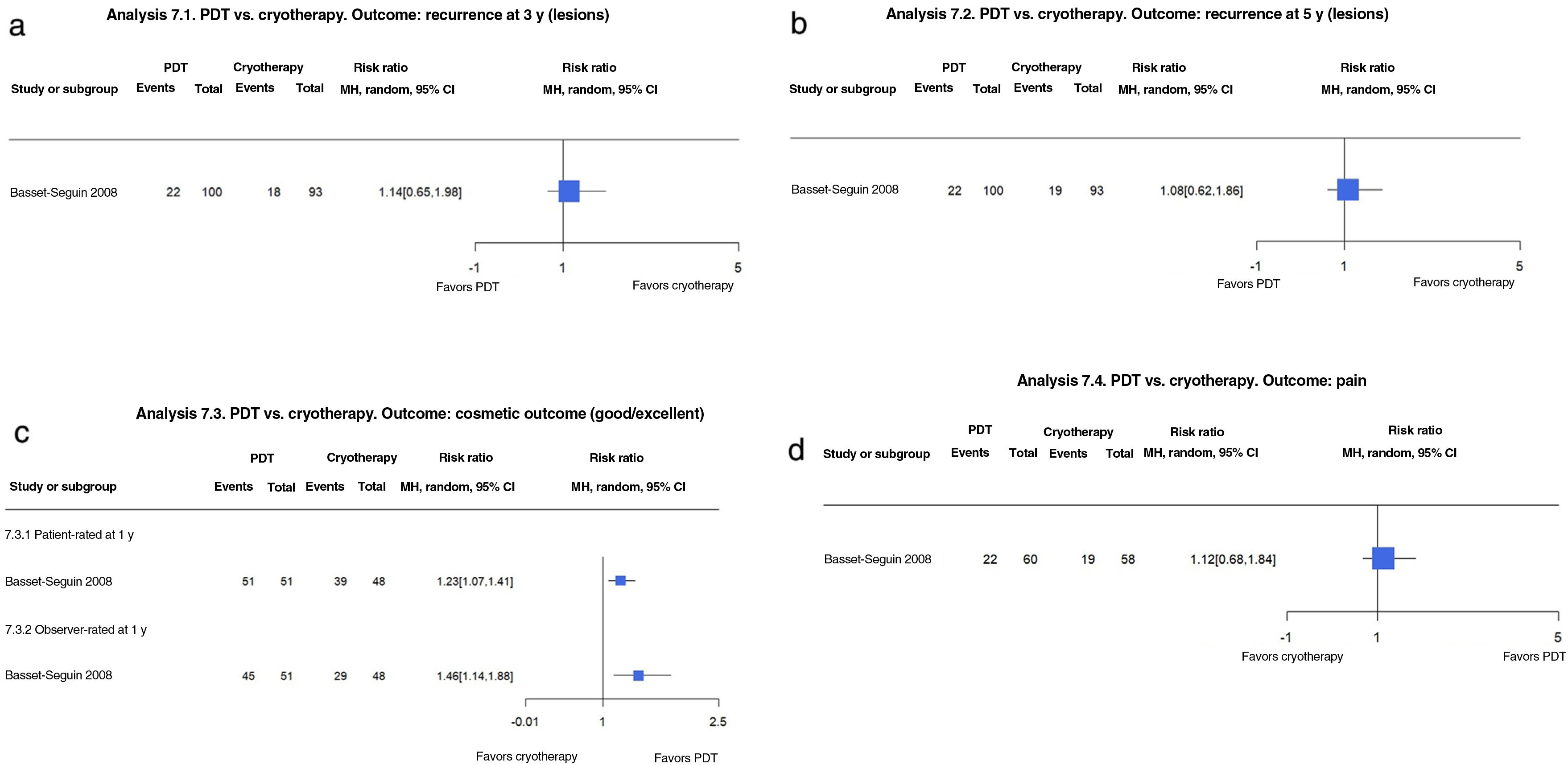
PDT vs. CryotherapyOn comparing MAL-PDT and cryotherapy in 118 participants with 219 BCCs followed for 5 years, Basset-Seguin et al.17 found no significant differences in recurrence at 3 years (22% vs. 19.4%; RR, 1.14; 95% CI, 0.65–1.98; low-certainty evidence) or 5 years (22% vs. 20%; RR, 1.08; 95% CI, 0.62–1.86; low-certainty evidence).1 The treatments also resulted in similar rates of pain (33% for MAL-PDT vs. 37% for cryotherapy; RR, 1.12; 95% CI, 0.68–1.84; low-certainty evidence)1,17 (Fig. 7A, B).
(7.1 and 7.2) Photodynamic therapy (PDT) vs. cryosurgery. Outcome: recurrence at 3 and 5 years, respectively (analyses 16.1 and 16.2 in the original review1). Interpretation: low evidence with just 1 study with a high risk of reporting bias for recurrence at 3 and 5 years. Inconclusive findings. (7.3) PDT vs. cryosurgery. Outcome: patient- and observer-rated cosmetic outcome (analysis 16.3 in the original review1). Interpretation: low-certainty evidence from just 1 study with a high risk of reporting bias for patient- and observer-rated cosmetic outcomes. The findings suggest that PDT results in better cosmetic outcomes than cryotherapy. (7.4) PDT vs. cryosurgery. Outcome: pain (analysis 16.4 in the original review1). Interpretation: low-certainty evidence from just 1 study with a high risk of reporting bias for pain. Confidence interval crossing 1, indicating inconclusive evidence on whether PDT causes more or less pain. In other words, the evidence is inconclusive.
MAL-PDT appears to result in higher rates of cosmetic outcomes rated as good or excellent by patients at 1 year (100% vs. 81.3% for cryotherapy; RR, 1.23; 95% CI, 1.07–1.41; moderate-certainty evidence).1,17 Similar findings were noted for observer-rated outcomes at 1 year, with 100% of MAL-PDT outcomes rated as good or excellent at 1 year compared with 61% of cryotherapy outcomes (RR, 1.46; 95% CI, 1.07–1.88, moderate-certainty evidence) (Fig. 7C).1,17 Wang et al.18 compared ALA-PDT with cryotherapy in 88 patients with nodular and superficial BCC. Risk of recurrence was not analyzed in the Cochrane review1 as the follow-up time was just 1 year. ALA-PDT also appears to be associated with an increased likelihood of good or excellent cosmetic outcomes compared with cryotherapy at 1 year (92.8% vs. 54.1%; RR, 1.72; 95% CI, 1.26–2.34; moderate-certainty evidence).
ALA-PDT is probably more painful than cryotherapy (difference in means, 11.00; 95% CI, 1.12–23.12; moderate-certainty evidence). In the study by Wang et al.,18 2 patients died of a cause unrelated to BCC or treatment. One patient treated with ALA-PDT described pain radiating from the treatment site, while another, treated with cryotherapy, developed a bacterial infection.1,18 (Fig. 7D).
Discussion and ConclusionsWe have summarized the results for 7 of the 52 comparisons made in the original Cochrane review of interventions for BCC of the skin by Thomson et al.1
Based on the available evidence, SE continues to be the most effective treatment for BCC, at least for low-risk histologic subtypes and tumors located in low-risk areas.1,19 Although MMS may result in fewer tumor recurrences at 3 to 5 years and fewer AEs, the findings are inconclusive as they are based on a single trial and have a wide CI. Radiotherapy, by contrast, appears to result in worse cosmetic outcomes and more recurrences than SE, but the evidence is uncertain as it is from a single study with a high risk of blinding bias and a wide CI, reflecting imprecision.
Within the group of nonsurgical treatments, imiquimod appears to be associated with more recurrences than SE, but it possibly results in better cosmetic outcomes. Again, the findings are inconclusive, as the evidence for recurrence at 3 and 5 years is of moderate certainty due to a wide CI and reliance on a single study with a risk of blinding bias.1,19
Imiquimod appears to be associated with a lower risk of recurrence compared with both MAL-PDT and 5-FU. Robust evidence indicating superior cosmetic outcomes for any of the treatments is lacking.1,19
SE appears to be associated with fewer recurrences at 3 years compared with MAL-PDT, but again, the evidence is uncertain. MAL-PDT, in turn, seems to result in better cosmetic outcomes, but the evidence is of moderate quality due to unclear blinding.1,19
Finally, the quality of the evidence suggesting that MAL-PDT results in fewer recurrences at 3 and 5 years compared with cryotherapy is low, as it is based on just 1 study. As with other treatments, it is also unclear whether or not MAL-PDT results in less pain and better cosmetic outcomes.1,19
Overall, the bulk of the evidence behind the results presented for each of the treatments is from single studies, preventing meta-analysis. Although most of the trials were multicenter studies, they involved small samples, explaining the wide CIs. They also had blinding issues, which will have affected subjective assessments of outcomes such as pain and cosmetic results. An additional problem identified in the Cochrane review was a lack of standardization in relation to recurrences and cosmetic outcomes that threatens not only the internal validity of the studies but also their external validity and reproducibility
FundingThis study received no external funding.
Conflict of InterestThe authors declare that they have no conflicts of interest.
We thank Dr. Hassiel Ramírez for his invaluable help with the translation into Spanish of the original Cochrane review.
This article is based on a Cochrane review published in the Cochrane Database of Systematic Reviews (CDSR) 2020, volume 11, Art. No.: CD003412. doi:10.1002/14651858.CD003412.pub3.



![(4.1) Photodynamic therapy with methylaminolevulinate (MAL-PDT) vs. surgical excision (SE). Outcome: recurrence at 3 years (analysis 10.1 in the original review1). Interpretation: low-certainty evidence from just 1 study with a high risk of bias and imprecision in the evaluation on the estimated effect of recurrence at 3 years (wide confidence interval [CI]). (4.2) MAL-PDT vs. SE. Outcome: good/excellent cosmetic outcome (analysis 10.2 in the original review1). Interpretation: moderate-certainty evidence in favor of MAL-PDT from a single study with a high risk of blinding bias, with narrow CIs for patient-rated outcomes and wide CIs (imprecision) for observer-rated outcomes. MH indicates Mantel–Haenszel. (4.1) Photodynamic therapy with methylaminolevulinate (MAL-PDT) vs. surgical excision (SE). Outcome: recurrence at 3 years (analysis 10.1 in the original review1). Interpretation: low-certainty evidence from just 1 study with a high risk of bias and imprecision in the evaluation on the estimated effect of recurrence at 3 years (wide confidence interval [CI]). (4.2) MAL-PDT vs. SE. Outcome: good/excellent cosmetic outcome (analysis 10.2 in the original review1). Interpretation: moderate-certainty evidence in favor of MAL-PDT from a single study with a high risk of blinding bias, with narrow CIs for patient-rated outcomes and wide CIs (imprecision) for observer-rated outcomes. MH indicates Mantel–Haenszel.](https://static.elsevier.es/multimedia/00017310/0000011400000001/v1_202301060625/S0001731022009358/v1_202301060625/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)