Tumor necrosis factor α (TNF) inhibitors are used to treat different inflammatory diseases. Although these biologics have an adequate safety profile, they have been associated with paradoxical reactions.
Material and methodsRetrospective review of patients on TNF inhibitor therapy who developed a paradoxical skin reaction and were seen at the dermatology department of Hospital Universitari Parc Taulí in Sabadell, Spain.
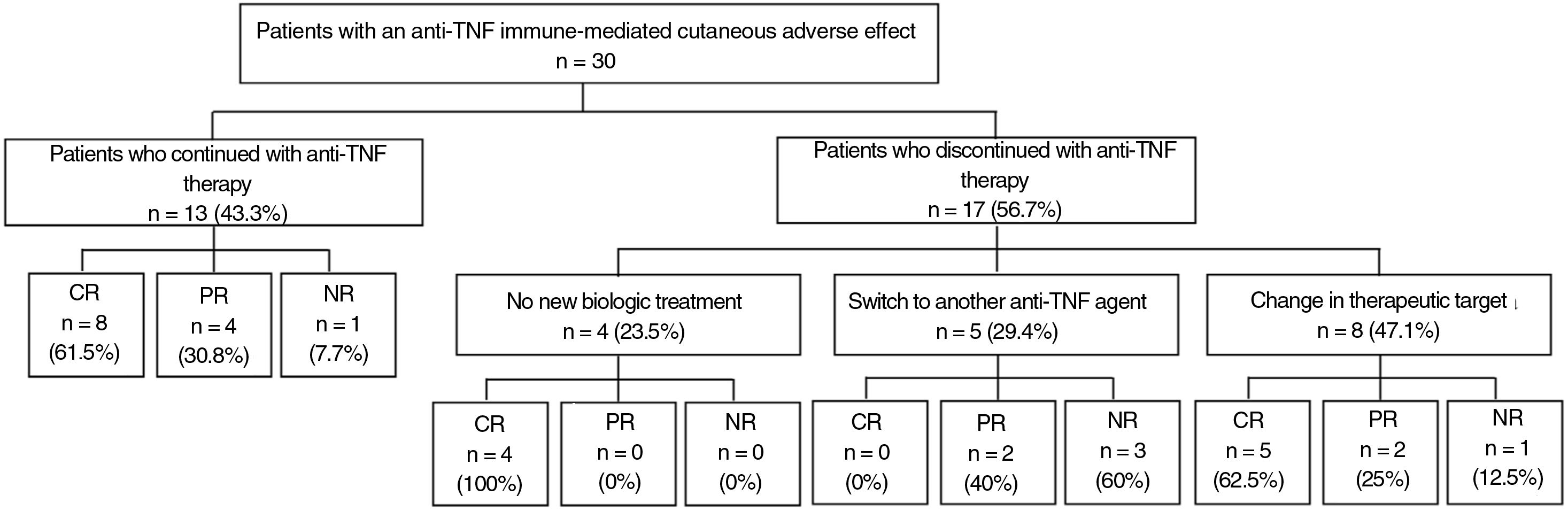
ResultsWe collected data on 30 patients under treatment with a TNF inhibitor who developed an immune-mediated skin reaction in the form of psoriasis (90%), alopecia (6.7%), or neutrophilic dermatitis (3.3%). The most common drugs involved were adalimumab (56.7%) and infliximab (40%). Psoriasiform reactions mostly manifested as generalized plaques (62.9%) or palmoplantar pustulosis (37%). Thirteen patients (43.3%) continued on the same TNF inhibitor and 12 of them (92.3%) achieved partial or complete resolution of lesions. Five patients were switched to a different TNF inhibitor, but none of them achieved complete resolution. Eight patients were switched to a biologic with a different target, and 5 of them (62.5%) achieved partial or complete resolution.
ConclusionsParadoxical reactions during TNF inhibitor therapy do not always require a change of treatment. In our series, the addition of a topical and/or systemic treatment resolved the skin lesions in more than half of the patients, and switching to a drug with a different target was more effective. A change of strategy should be contemplated in more serious cases.
Los fármacos biológicos inhibidores del factor de necrosis tumoral (TNF) alfa son usados para tratar diferentes enfermedades inflamatorias. A pesar de su adecuado perfil de seguridad, se han descrito reacciones paradójicas asociadas a estos tratamientos.
Material y métodoSe ha realizado una revisión retrospectiva de los pacientes en tratamiento con un anti-TNF que hubiesen presentado una reacción paradójica con afectación cutánea visitados en el Servicio de Dermatología del Hospital Universitari Parc Taulí de Sabadell.
ResultadosRegistramos 30 pacientes en tratamiento con un anti-TNF que desarrollaron un efecto adverso cutáneo inmunomediado en forma de psoriasis (90%), alopecia (6,7%) o dermatitis neutrofílica (3,3%). Adalimumab fue el fármaco más implicado (56,7%), seguido de infliximab (40%). La morfología de la reacción psoriasiforme más descrita es la generalizada en placas (62,9%), seguida de la pustulosis palmo-plantar (37%). El 43,3% de los pacientes mantuvieron el anti-TNF, y de ellos el 92,3% obtuvieron una resolución total y parcial. De los 5 pacientes que iniciaron otro anti-TNF, ninguno obtuvo una resolución total. De los 8 pacientes que cambiaron a un tratamiento biológico diferente al anti-TNF, el 62,5% obtuvieron una resolución total o parcial.
DiscusiónLa aparición de una reacción paradójica no siempre obliga al cambio de tratamiento biológico, puesto que se ha observado la resolución de las lesiones cutáneas con un tratamiento tópico y/o sistémico adicional en más de la mitad de los pacientes, sin necesidad de suspender el anti-TNF. Si la afectación es grave, se debe plantear el cambio de tratamiento biológico, siendo más eficaz iniciar un fármaco dirigido a una diana terapéutica distinta al anti-TNF.
Tumor necrosis factor (TNF) alfa inhibitors are used to treat different inflammatory diseases, such as inflammatory bowel disease, psoriasis, hidradenitis suppurativa (HS), arthritis, and spondyloarthritis, among others. Despite having a good safety profile, different adverse effects have been reported, with skin involvement in the form of injection site reaction, skin infection, or immune-mediated cutaneous adverse effects (ICAEs) being frequent. Some of the immune-mediated cutaneous adverse effects reported include psoriasis, lichen planus, systemic lupus erythematosus or drug-induced lupus, HS, bullous pemphigoid, vasculitis, cutaneous sarcoidosis, vitiligo, and acneiform rash, among others.1–5 When these immune-mediated diseases respond to the same biologic agent, these are denoted paradoxical reactions (PR), defined as the onset or worsening of one of the diseases that is treated with these medications.3–8 The range of PRs resulting from treatment with anti-TNF agents is extensive, although skin involvement in the form of psoriasiform reaction is the most widely reported.4
Discontinuing the drug or switching to another biologic agent leads to resolution of the skin lesions in most cases, but at times, good control can be achieved with additional treatment, thus allowing anti-TNF treatment to be maintained (Fig. 1).
A pathophysiological explanation for these reactions has still not been established; likewise, there are no therapeutic guidelines for appropriate management of these patients.
The objective of this study was to retrospectively review the clinical characteristics, outcomes, and treatment of patients attended in our center who developed an ICAE caused by anti-TNF treatment.
Material and MethodsWe conducted a descriptive, retrospective study of all patients attended in the dermatology department of the Hospital Universitari Parc Taulí, Sabadell, Spain, between January 2006 and December 2022, in treatment with anti-TNF agents who had developed an ICAE, using the registry of a database maintained by the digestive, rheumatology, and dermatology departments of our hospital.
The following variables were collected for each patient: sex, age, personal and family history of psoriasis, underlying disease for which anti-TNF agents were prescribed (Crohn disease, ulcerative colitis, hidradenitis suppurativa, ankylosing spondylitis, and recurring chronic multifocal osteomyelitis), biologic agent used at the time of the ICAE (adalimumab, infliximab, and golimumab), prior and concomitant treatments, latency period from initiating the anti-TNF agent until onset of the ICAE, type of ICAE (psoriasis, alopecia, neutrophilic dermatosis), morphology of the ICAE, histological confirmation, additional treatment for the ICAE (topical and systemic), anti-TNF discontinuation, new biologic treatment, and resolution of the skin lesions (complete, partial, or no resolution). The morphology of the psoriasiform reactions was classified into 6 types: generalized plaque type, palmoplantar pustulosis, inverted, scalp, guttate, and erythrodermic. The types of psoriasiform reactions were not mutually exclusive, and the same patient could be diagnosed with several different types of clinical reaction.
The data were complied in tables for analysis using the SPSS program. Subsequently, a descriptive analysis was performed with median and range for quantitative variables and frequencies for qualitative variables.
ResultsThirty patients were included (Table 1), of whom 20 (66.7%) were women and 10 (33.3%) were men. Their age ranged from 15 to 65 years, with a median age of 49 years. Five patients (16.7%) had a personal history of psoriasis and 2 (6.7%) had a family history of the condition.
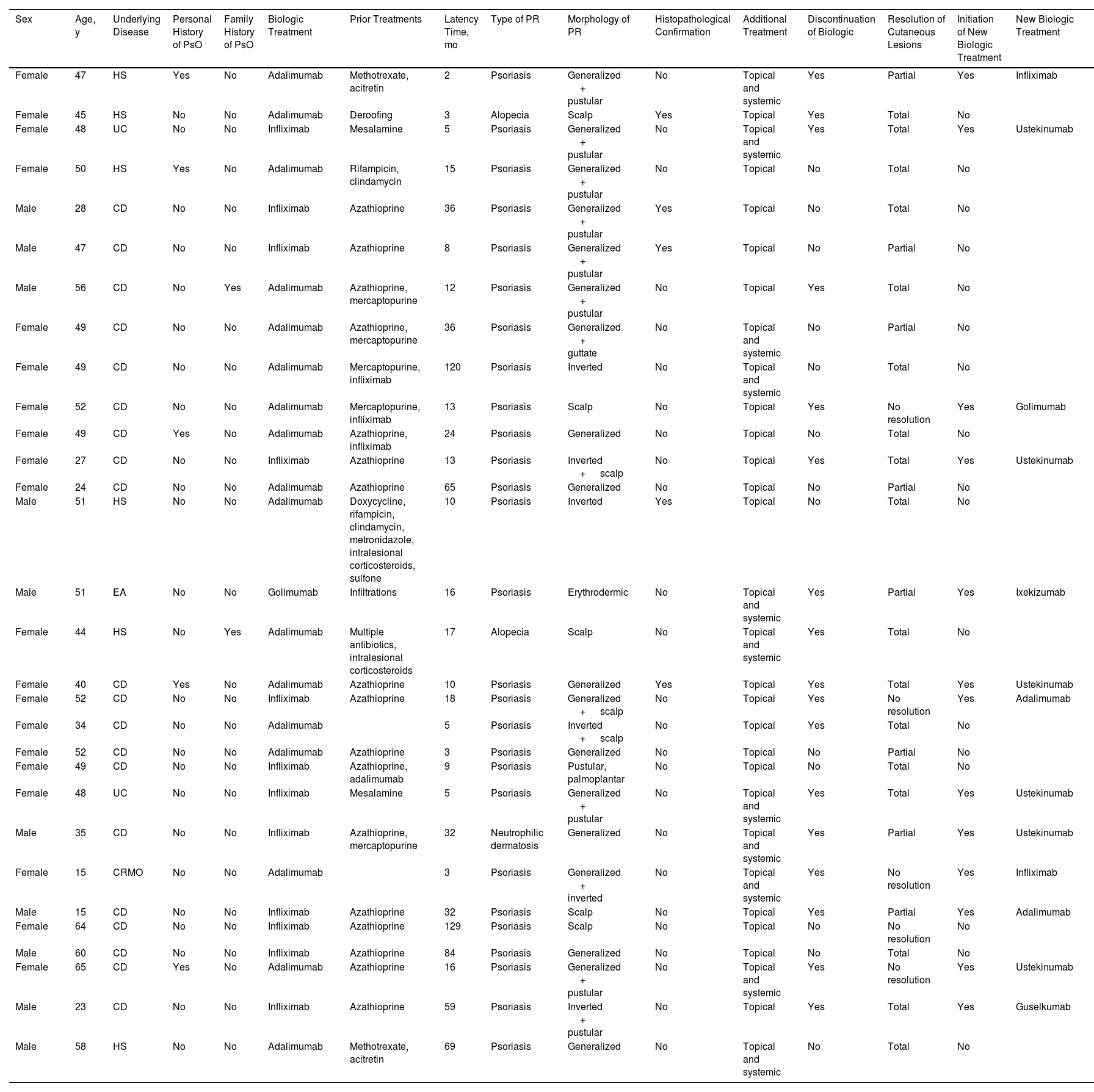
Data from Patients in Our Series who Presented an Anti-TNF Immune-Mediated Cutaneous Adverse Effect.
| Sex | Age, y | Underlying Disease | Personal History of PsO | Family History of PsO | Biologic Treatment | Prior Treatments | Latency Time, mo | Type of PR | Morphology of PR | Histopathological Confirmation | Additional Treatment | Discontinuation of Biologic | Resolution of Cutaneous Lesions | Initiation of New Biologic Treatment | New Biologic Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | 47 | HS | Yes | No | Adalimumab | Methotrexate, acitretin | 2 | Psoriasis | Generalized + pustular | No | Topical and systemic | Yes | Partial | Yes | Infliximab |
| Female | 45 | HS | No | No | Adalimumab | Deroofing | 3 | Alopecia | Scalp | Yes | Topical | Yes | Total | No | |
| Female | 48 | UC | No | No | Infliximab | Mesalamine | 5 | Psoriasis | Generalized + pustular | No | Topical and systemic | Yes | Total | Yes | Ustekinumab |
| Female | 50 | HS | Yes | No | Adalimumab | Rifampicin, clindamycin | 15 | Psoriasis | Generalized + pustular | No | Topical | No | Total | No | |
| Male | 28 | CD | No | No | Infliximab | Azathioprine | 36 | Psoriasis | Generalized + pustular | Yes | Topical | No | Total | No | |
| Male | 47 | CD | No | No | Infliximab | Azathioprine | 8 | Psoriasis | Generalized + pustular | Yes | Topical | No | Partial | No | |
| Male | 56 | CD | No | Yes | Adalimumab | Azathioprine, mercaptopurine | 12 | Psoriasis | Generalized + pustular | No | Topical | Yes | Total | No | |
| Female | 49 | CD | No | No | Adalimumab | Azathioprine, mercaptopurine | 36 | Psoriasis | Generalized + guttate | No | Topical and systemic | No | Partial | No | |
| Female | 49 | CD | No | No | Adalimumab | Mercaptopurine, infliximab | 120 | Psoriasis | Inverted | No | Topical and systemic | No | Total | No | |
| Female | 52 | CD | No | No | Adalimumab | Mercaptopurine, infliximab | 13 | Psoriasis | Scalp | No | Topical | Yes | No resolution | Yes | Golimumab |
| Female | 49 | CD | Yes | No | Adalimumab | Azathioprine, infliximab | 24 | Psoriasis | Generalized | No | Topical | No | Total | No | |
| Female | 27 | CD | No | No | Infliximab | Azathioprine | 13 | Psoriasis | Inverted + scalp | No | Topical | Yes | Total | Yes | Ustekinumab |
| Female | 24 | CD | No | No | Adalimumab | Azathioprine | 65 | Psoriasis | Generalized | No | Topical | No | Partial | No | |
| Male | 51 | HS | No | No | Adalimumab | Doxycycline, rifampicin, clindamycin, metronidazole, intralesional corticosteroids, sulfone | 10 | Psoriasis | Inverted | Yes | Topical | No | Total | No | |
| Male | 51 | EA | No | No | Golimumab | Infiltrations | 16 | Psoriasis | Erythrodermic | No | Topical and systemic | Yes | Partial | Yes | Ixekizumab |
| Female | 44 | HS | No | Yes | Adalimumab | Multiple antibiotics, intralesional corticosteroids | 17 | Alopecia | Scalp | No | Topical and systemic | Yes | Total | No | |
| Female | 40 | CD | Yes | No | Adalimumab | Azathioprine | 10 | Psoriasis | Generalized | Yes | Topical | Yes | Total | Yes | Ustekinumab |
| Female | 52 | CD | No | No | Infliximab | Azathioprine | 18 | Psoriasis | Generalized + scalp | No | Topical | Yes | No resolution | Yes | Adalimumab |
| Female | 34 | CD | No | No | Adalimumab | 5 | Psoriasis | Inverted + scalp | No | Topical | Yes | Total | No | ||
| Female | 52 | CD | No | No | Adalimumab | Azathioprine | 3 | Psoriasis | Generalized | No | Topical | No | Partial | No | |
| Female | 49 | CD | No | No | Infliximab | Azathioprine, adalimumab | 9 | Psoriasis | Pustular, palmoplantar | No | Topical | No | Total | No | |
| Female | 48 | UC | No | No | Infliximab | Mesalamine | 5 | Psoriasis | Generalized + pustular | No | Topical and systemic | Yes | Total | Yes | Ustekinumab |
| Male | 35 | CD | No | No | Infliximab | Azathioprine, mercaptopurine | 32 | Neutrophilic dermatosis | Generalized | No | Topical and systemic | Yes | Partial | Yes | Ustekinumab |
| Female | 15 | CRMO | No | No | Adalimumab | 3 | Psoriasis | Generalized + inverted | No | Topical and systemic | Yes | No resolution | Yes | Infliximab | |
| Male | 15 | CD | No | No | Infliximab | Azathioprine | 32 | Psoriasis | Scalp | No | Topical | Yes | Partial | Yes | Adalimumab |
| Female | 64 | CD | No | No | Infliximab | Azathioprine | 129 | Psoriasis | Scalp | No | Topical | No | No resolution | No | |
| Male | 60 | CD | No | No | Infliximab | Azathioprine | 84 | Psoriasis | Generalized | No | Topical | No | Total | No | |
| Female | 65 | CD | Yes | No | Adalimumab | Azathioprine | 16 | Psoriasis | Generalized + pustular | No | Topical and systemic | Yes | No resolution | Yes | Ustekinumab |
| Male | 23 | CD | No | No | Infliximab | Azathioprine | 59 | Psoriasis | Inverted + pustular | No | Topical | Yes | Total | Yes | Guselkumab |
| Male | 58 | HS | No | No | Adalimumab | Methotrexate, acitretin | 69 | Psoriasis | Generalized | No | Topical and systemic | No | Total | No |
Abbreviations: AS, ankylosing spondylitis; CD, Crohn disease; CRMO, chronic recurrent multifocal osteomyelitis; HS, hidradenitis suppurativa; PsO, psoriasis; PR, paradoxical reaction; UC, ulcerative colitis.
The underlying diseases for which anti-TNF treatment was administered were Crohn disease in 20 (66.7%), ulcerative colitis in 2 (6.7%), hidradenitis suppurativa in 6 (20%), ankylosing spondylitis in 1 (3.3%), and recurring chronic multifocal osteomyelitis (RCMO) in 1 (3.3%).
The majority of patients had received prior immunosuppressants (methotrexate, azathioprine, corticosteroids, among others). Prior treatments did not appear to have influenced the onset of ICAEs.
In our review, 3 agents were implicated in the onset of an ICAE: adalimumab was associated with such reactions in 17 cases (56.7%), followed by infliximab in 12 (40%), and golimumab in 1 (3.3%).
The most frequent form of presentation of an ICAE was paradoxical psoriasiform reaction in 27 (90%) patients. The morphology of psoriasiform reaction was variable. Generalized plaque psoriasis was reported in 17 cases (62.9%), palmoplantar pustulosis in 10 (37%), inverted psoriasis in 6 (22.2%), scalp psoriasis in 6 (22.2%), guttate psoriasis in 1 (3.7%), and erythrodermic psoriasis in 1 (3.7%). In most cases, 2 or more forms of psoriasiform reaction presented together, in particular, the combination of generalized plaques with palmoplantar pustulosis.
Other immune-mediated adverse cutaneous effects reported in the patient review were alopecia in 2 cases (6.7%)—in form of alopecia areata in 1 patient and inflammatory neutrophilic alopecia in the other patient—and neutrophilic dermatitis in 1 (3.3%) in the form of acute generalized exanthematous pustulosis.
The latency period from initiation of biologic treatment until onset of the ICAE was very variable, with a median of 15.5 months and a range of 2 to 129 months.
A biopsy of the skin lesions was performed in 6 patients. The pathology findings confirmed diagnosis of psoriasis in 4, neutrophilic alopecia in 1, and neutrophilic dermatitis in another patient. There were no histological differences between paradoxical psoriasis and spontaneous psoriasis.
Additional treatment was initiated with topical corticosteroids in all patients, with systemic treatment (methotrexate, acitretin, and/or phototherapy) in 11 (36.7%).
At the physician's discretion, anti-TNF treatment was maintained in 13 patients (43.3%); 8 of these (61.5%) had complete resolution; 4 (30.8%) partial resolution, and 1 (7.7%) did not improve.
The treating physician decided to discontinue the anti-TNF agent in 17 patients (56.7%) due to the ICAE. Of these, 4 did not start another biologic treatment and in all cases, complete resolution of the lesions was reported after discontinuation. The remaining 13 patients started another biologic treatment. Five initiated treatment with a different anti-TNF agent: infliximab (n=2), adalimumab (n=2), and golimumab (n=1). Among these patients, none achieved complete resolution while 2 (40%) achieved partial resolution, and 3 (60%) did not show any improvement in skin lesions. Eight patients initiated biologic treatment directed against targets other than TNF. Of the 6 patients who initiated treatment with ustekinumab, 4 (66.6%) achieved complete resolution, 1 (16.7%) achieved partial resolution, and 1 (16.7%) did not achieve any response; this latter patient had a personal history of psoriasis. One patient initiated ixekizumab with partial resolution and another patient started guselkumab with complete resolution (Table 1).
DiscussionSince 2004, when the first case of PR associated with infliximab was reported in a patient with Crohn disease, many studies have described the onset of PRs with anti-TNF agents.9 Psoriasiform-type PRs have been observed in between 2% and 5% of patients in treatment with anti-TNF agents.10 However, Almutairi et al.9 reported an incidence as high as 10.9% in patients with inflammatory bowel disease, most of whom had Crohn disease.11
Other possible immune-mediated adverse cutaneous effects reported both in our case review and in other studies are alopecia and neutrophilic dermatitis.3,4
Worsening of psoriasis or a change in morphology have also been reported as a type of PR,4,5,11 but in our series, we did not include patients in treatment with anti-TNF for psoriasis as the underlying condition and who could potentially have presented a PR.
The demographic characteristics of our patients were comparable with those observed in other studies.3,6,11,12 Most patients who present an ICAE were women, an observation that could be related to the epidemiology of the underlying inflammatory conditions, as these are more prevalent in females. No differences were observed in terms of age, and most patients did not have a personal or family history of psoriasis.
The time to onset of an ICAE was very variable, and so it is not possible to establish a fixed latency period.6,9 Likewise, no relationship has been established between ICAEs and prior therapy or therapy administered concomitantly with the anti-TNF agent for the underlying condition.3
In this review, we have reported an ICAE with adalimumab, infliximab, and golimumab, with the former of these being the most frequently responsible. However, other studies have found that the most frequently implicated drug is infliximab. The greater implication of adalimumab could be explained by its more widespread use in clinical practice in our hospital. ICAEs have also been reported with other anti-TNF agents, such as etanercept and certolizumab, drugs that were not included in our case review.3,5,6,9,11,13
With regards the morphology of the ICAE, as in our findings, psoriasiform reaction clearly stands out, with predominance of generalized plaques (62.9%), followed by palmoplantar pustular form (37%), in agreement with other series.6,9,11 The systematic review by Brown et al.12 also reported the plaque morphology (44.8%) to be the most frequent, followed by the palmoplantar pustular form (36.3%). As in our study, cases of inverted, guttate and erythrodermic psoriasis and psoriasis with scalp involvement have also been reported. However, in most cases, different morphologies can be observed in the same patient, as observed in our series.5,6,9,11
In our review, we observe that the approach of the physician has been, in most cases, one of not performing a biopsy of skin lesions, probably because of previous literature reports that there are no histological differences between paradoxical psoriasis and spontaneous psoriasis, as we also observed. Nevertheless, Munera-Campos et al.3 comment that the presence of eosinophils or plasma cells could favor onset of drug-induced reactions. We are of the opinion that skin biopsy does not provide additional information for diagnosis of psoriasiform type PR or change any therapeutic decisions. It would only be justified in cases in which a nonpsoriasiform morphology was present or if there were doubts about the diagnosis.
In our review, it is observed that 92.3% of patients who maintained biologic treatment presented total or partial resolution with associated topical and/or systemic treatment, without the need to discontinue anti-TNF agents. When anti-TNF agents were suspended without initiating another biologic agent, all patients showed complete resolution, and so we suggest there is is no need to be too hasty when discontinuing the anti-TNF agent; this can be maintained in the event of mild and tolerable cutaneous reactions, particularly when there is good control of the underlying disease for which the biologic treatment is indicated.3,10,11
A switch to another anti-TNF agent is possible, but we have not observed resolution in any cases, and as many as 60% did not improve with the new treatment. These data are in agreement with the review by Puig,5 who described recurrence of psoriasiform lesions in 50% to 100% of patients treated with a second anti-TNF agent.
Alternatively, with the change to a drug with a different mechanism of action, complete resolution was reported in up to 62.5% of patients and so, if the physician decides to discontinue anti-TNF therapy and initiate another biologic therapy, it is recommended to start with a biologic with a target other than TNF.3,9,11
As reported by Wu and Smogorzewski,8 switching to ustekinumab could be a safe and effective option. In our review, although the sample size was small, no differences were observed in the percentage of patients with complete resolution among the group who maintained anti-TNF treatment and the group who switched to ustekinumab (61.5% vs. 66.6%). Moreover, the percentage of patients with partial resolution among those who maintained anti-TNF treatment is higher compared with those who switched to ustekinumab (30.8% vs. 16.7%). This observation could be explained by the tendency of the physician to maintain anti-TNF therapy in those patients with mild cutaneous reactions, and so the burden of skin disease could be considered lower in this group, leading to a better response to topical and/or systemic treatment.
However, there are still no therapeutic guidelines for management of ICAEs associated with biologic treatment. Likewise, there are also disagreements about whether the drug should be discontinued or not to achieve complete resolution of the lesions.6 Therefore, for the time being, therapeutic management is performed on an individual basis.
The limitations of this case series review are that data were collected in a single center, that this was a descriptive and retrospective study, and that the sample size analyzed was small. In addition, data were not collected for patients in treatment with anti-TNF agents for rheumatoid arthritis, perhaps because the rheumatologists in our center have not created a registry for ICAEs or because, in their experience, they treat the patients without referring them to the dermatology department. Another of the limitations is that the severity of the skin lesions was not recorded. In the case of psoriasiform PR, the PASI, BSA, and PGA scales could have been used, although application is difficult because of the atypical morphology of these types of reaction.
In conclusion, the ICAEs are known cutaneous reactions to treatment with anti-TNF agents. Our data show that, in a high percentage of patients who had an ICAE, complete or partial resolution of the cutaneous lesions was achieved with additional treatment, without the need to discontinue anti-TNF therapy. Nevertheless, it is important to assess the severity of the lesions to determine the most appropriate therapeutic approach. In the event of switching biologics, it was found that it is more effective to switch to drugs with a different therapeutic target.
Due to the administrative regulations that encourage the use of anti-TNF biosimilars, an increase in the use of these drugs has been observed, and so an increase in the incidence of case of ICAEs is to be expected. Thus, continuous monitoring of patients in treatment with anti-TNF agents is recommended to detect possible ICAEs, as these lesions can become manifest at any time.
In summary, we conclude that discontinuation of anti-TNF therapy should only be considered in cases of severe ICAEs, in lesions resistant to additional treatment, or when there is a major impact on quality of life.
Conflicts of InterestDr. Miquel Ribera has received assistance and payments related to investigation, consulting, and training from the following companies: AbbVie, Almirall, Amgen, Gebro Pharma, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Pierre-Fabre, Sandoz, SKB, and UCB. Dr. Romany has participated in clinical trials, conferences, and steering committees with the following pharmaceutical companies: AbbVie, Almirall, Boehringuer-Ingelheim, Janssen, LEO Pharma, Lilly, Novartis, Sanofi, and UCB.