Recognition of the typical histopathological findings of mycosis fungoides (MF) can help clinicians establish diagnosis and is of prognostic value for disease progression and treatment response. Although the histopathological characteristics of MF have been recorded since the earliest descriptions, peculiarities within the Colombian population have not been explored to date. Our objective was to describe the histopathology of MF in biopsy samples obtained at the Dermatopathology Laboratory of the University of Antioquia, Medellín, Colombia.
We reviewed all biopsy data collected at the Dermatopathology Laboratory of the University of Antioquia between March 1976 and January 2013. These results corresponded to clinical data and hematoxylin–eosin staining from 252 preselected confirmed diagnoses of cutaneous T-cell lymphoma, MF, parapsoriasis, and follicular mucinosis. Other lymphomas, Sézary syndrome, and post-treatment biopsies were excluded from our analysis. Of the selected samples, 90 corresponded to a diagnosis of MF. Data on HTLV 1 status were not available for any of the cases analyzed.
The median age (available in 87 of 90 cases) was 52 years, which is lower than that reported in the United States and Japan (58 and 55 y, respectively).1,2 Time to diagnosis was 3.8 years (range, 1 mo to 20 y), which is similar to that reported in recent studies. Strikingly, in 4 cases the reported clinical lesions were papules, and folliculotropism was described in only 1 case. Papules could be considered an initial lesion in the progression towards plaque formation. However, a clinical variant of papular MF has been described.3
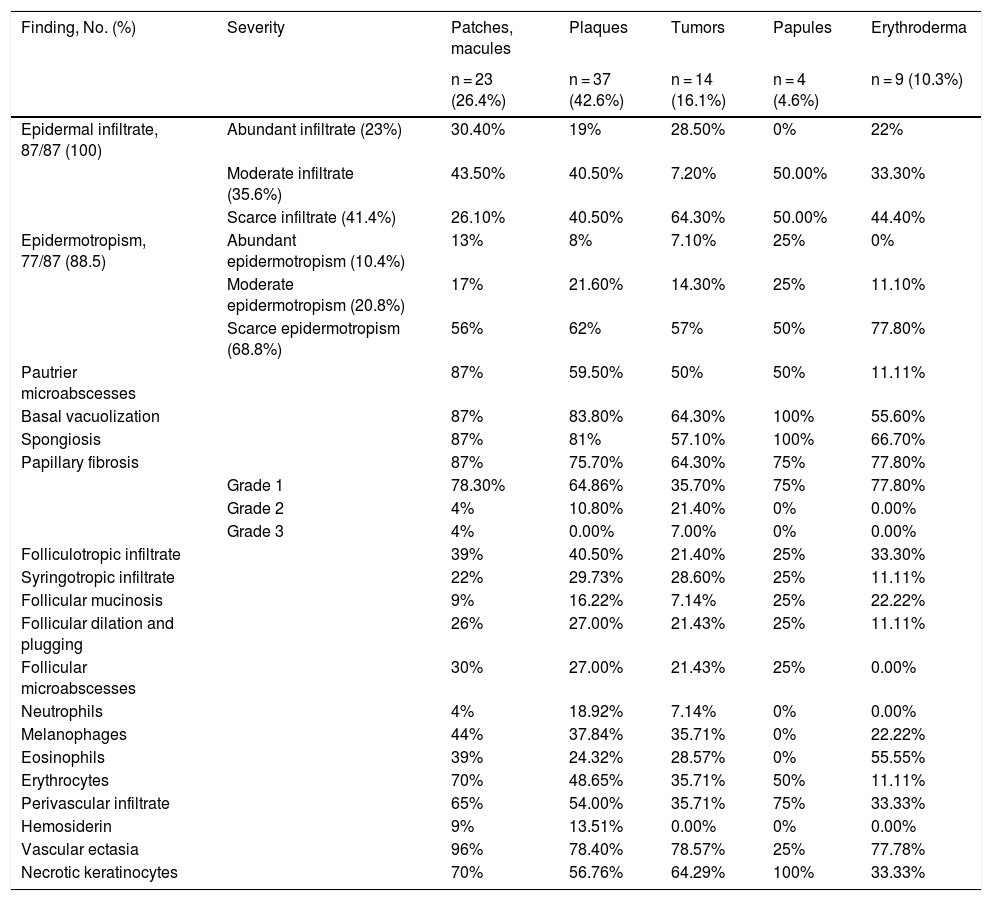
The histopathological findings according to clinical lesion type are described in Table 1. Infiltrate was defined as follows: abundant, >50% infiltration of the dermoepidermal junction by atypical lymphocytes; moderate, 10–50% infiltration; scarce, <10% infiltration. Epidermotropism (evaluated at ×40 magnification) was defined as follows: abundant, >10 lymphocytes in the epidermis; moderate, 6–10 lymphocytes; scarce, 1–5 lymphocytes. Pautrier microabscesses were defined as the presence of at least 4 atypical lymphocytes in an intraepidermal vacuole. Papillary laminar fibrosis was defined as follows: grade 1, <10%; grade 2, 10–50%; and grade 3, >50%. These criteria were established by the group of dermatopathologists, given the absence of quantitative parameters in the literature.
Histopathological Findings According to Lesion Type: Patches and Macules, Plaques, and Tumors.
| Finding, No. (%) | Severity | Patches, macules | Plaques | Tumors | Papules | Erythroderma |
|---|---|---|---|---|---|---|
| n = 23 (26.4%) | n = 37 (42.6%) | n = 14 (16.1%) | n = 4 (4.6%) | n = 9 (10.3%) | ||
| Epidermal infiltrate, 87/87 (100) | Abundant infiltrate (23%) | 30.40% | 19% | 28.50% | 0% | 22% |
| Moderate infiltrate (35.6%) | 43.50% | 40.50% | 7.20% | 50.00% | 33.30% | |
| Scarce infiltrate (41.4%) | 26.10% | 40.50% | 64.30% | 50.00% | 44.40% | |
| Epidermotropism, 77/87 (88.5) | Abundant epidermotropism (10.4%) | 13% | 8% | 7.10% | 25% | 0% |
| Moderate epidermotropism (20.8%) | 17% | 21.60% | 14.30% | 25% | 11.10% | |
| Scarce epidermotropism (68.8%) | 56% | 62% | 57% | 50% | 77.80% | |
| Pautrier microabscesses | 87% | 59.50% | 50% | 50% | 11.11% | |
| Basal vacuolization | 87% | 83.80% | 64.30% | 100% | 55.60% | |
| Spongiosis | 87% | 81% | 57.10% | 100% | 66.70% | |
| Papillary fibrosis | 87% | 75.70% | 64.30% | 75% | 77.80% | |
| Grade 1 | 78.30% | 64.86% | 35.70% | 75% | 77.80% | |
| Grade 2 | 4% | 10.80% | 21.40% | 0% | 0.00% | |
| Grade 3 | 4% | 0.00% | 7.00% | 0% | 0.00% | |
| Folliculotropic infiltrate | 39% | 40.50% | 21.40% | 25% | 33.30% | |
| Syringotropic infiltrate | 22% | 29.73% | 28.60% | 25% | 11.11% | |
| Follicular mucinosis | 9% | 16.22% | 7.14% | 25% | 22.22% | |
| Follicular dilation and plugging | 26% | 27.00% | 21.43% | 25% | 11.11% | |
| Follicular microabscesses | 30% | 27.00% | 21.43% | 25% | 0.00% | |
| Neutrophils | 4% | 18.92% | 7.14% | 0% | 0.00% | |
| Melanophages | 44% | 37.84% | 35.71% | 0% | 22.22% | |
| Eosinophils | 39% | 24.32% | 28.57% | 0% | 55.55% | |
| Erythrocytes | 70% | 48.65% | 35.71% | 50% | 11.11% | |
| Perivascular infiltrate | 65% | 54.00% | 35.71% | 75% | 33.33% | |
| Hemosiderin | 9% | 13.51% | 0.00% | 0% | 0.00% | |
| Vascular ectasia | 96% | 78.40% | 78.57% | 25% | 77.78% | |
| Necrotic keratinocytes | 70% | 56.76% | 64.29% | 100% | 33.33% |
Epidermotropism has been considered a histopathological marker of MF and has been reported in more than 75% of cases.4 In our study it was detected in 90% of cases. Pautrier microabscesses were more frequent (61% of cases). Other authors have described this finding in 29%–37% of cases.5,6
Although MF has traditionally been described as the presence of epidermotropic lymphocytes in the absence of spongiosis,7 we frequently observed areas of vacuolization of the basal layer and spongiosis associated with or accompanied by lymphocyte marginalization. Marginalization and spongiosis were observed in 76% of cases (n = 68), and marginalization and vacuolization in 78% of cases (n = 70). Spongiosis and vacuolization of the basal cell layer have been described by other authors in up to 86% of cases.7 We do not consider these findings sufficient to rule out MF. The biological significance of these histological findings remains to be determined. It may also be useful to clinically evaluate patients for superimposed contact dermatitis and, in positive cases, to remove all stimuli that potentially contribute to increased inflammatory and tumoral activity.
Accompanying cells include eosinophils (reported in 12%–54% of cases) and plasma cells (4%–38% of cases).8,9 In our population we observed accompanying eosinophils and plasma cells in 31% and 29% of cases, respectively.
Folliculotropism was observed in 35% of the reviewed biopsies. The highest incidence reported in other series was in Norwegian patients (21%), although most authors report this finding in 10% of cases.10
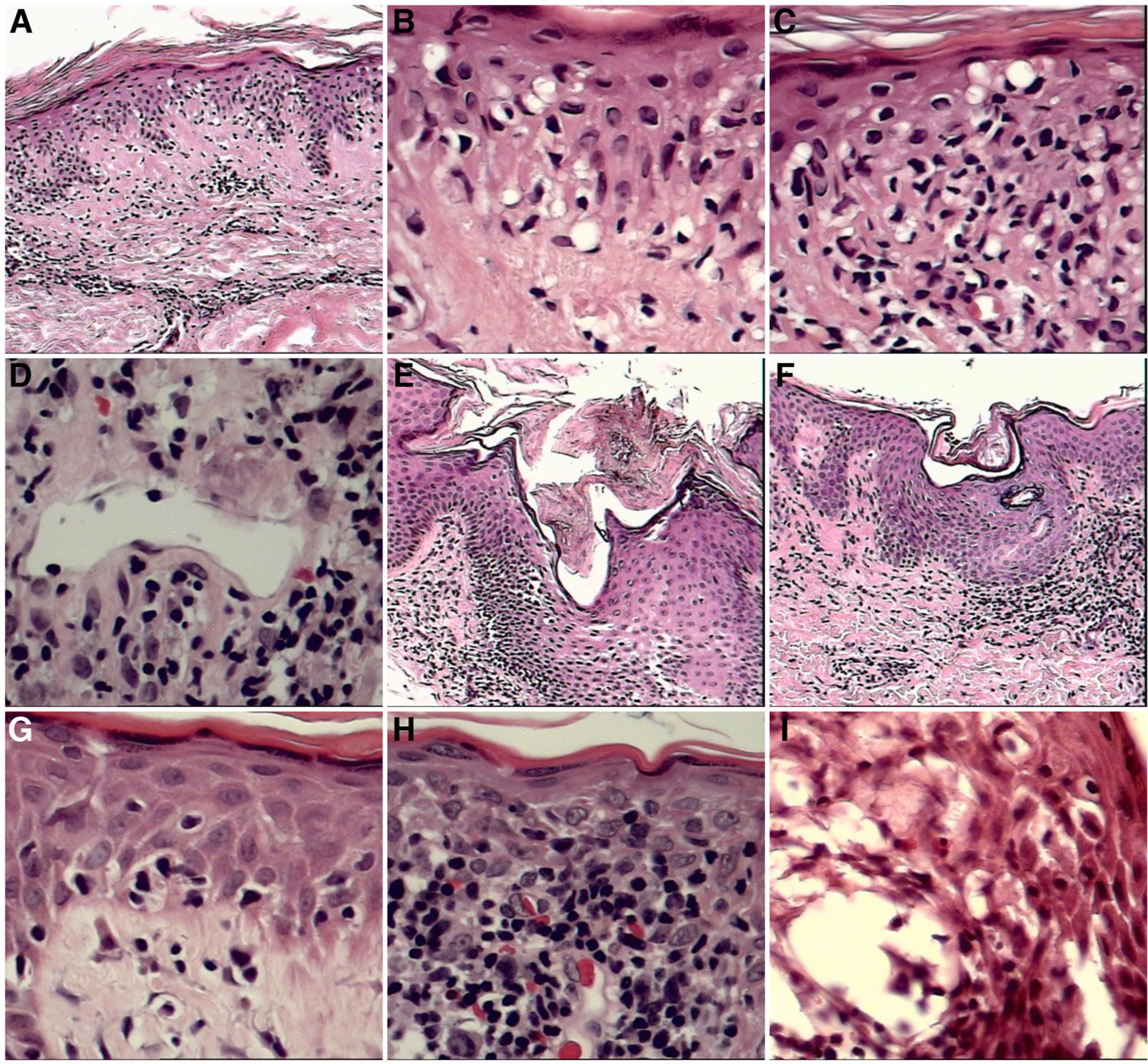
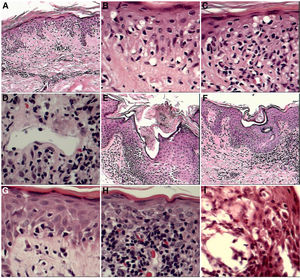
Certain histopathological findings varied according to clinical lesion type. This was most notable for Pautrier microabscesses, which were present in 87% of patch- and plaque-type lesions, but in only 50% of tumors. Basal layer vacuolization and spongiosis were also more common in patches and plaques than in tumors (Fig. 1).
A–C, Little reported but important findings. A, Extensive vacuolization of the basal layer (×10 magnification). B, Detail of vacuolization of the basal layer (×10). C, Vacuolization of the basal layer with effacement of the interface by atypical lymphocytes (×10). D–I, Other frequent findings. D, Vascular ectasia was observed in all stages of MF (×10). E, Dilation with corneal plugging of the infundibula (×10). F, Dilation with corneal plugging of the pores of the acrosyringia (×10). G, Extensive vacuolization of the basal layer (×10). H, Erythrocyte extravasation (×10). I, Spongiosis with microvesiculation associated with eosinophil exocytosis (×10), papules, and erythroderma.
In our population, in addition to the defining features of MF, we regularly observed foci of spongiosis and vacuolization of the basal layer, as well as folliculotropism. These findings may be related to the exposure of patients to exogenous agents that may participate in or exacerbate the immunopathogenic process in MF.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Mejia MN, Valencia Ocampo OJ, Correa LA, Wolff JC, Correa S, Velásquez Lopera MM. Histopatología de micosis fungoide en una población colombiana. Identificando las características de la micosis fungoide en poblaciones suramericanas. Actas Dermosifiliogr. 2022;113:91–94.