Hidradenitis suppurativa (HS) typically appears between the 2nd and 3rd decades of life and remains active, on a chronic basis, with some fluctuations. Although there are no prospective studies supporting it, improvement after menopause has been reported.1 There is wide variability in estimates of HS prevalence in individuals older than 65 years. A recent study, which sought related diagnoses and contacted patients to confirm the diagnosis, estimates a global prevalence of 0.77% (0.4% in patients older than 65 years).2 However, studies based on coded diagnoses from large insurance databases suggest a lower frequency of 0.05% in patients older than 65 years.3,4
The possibility of finding advanced disease in older patients, as expected in a progressive condition, and the difficulty associated with managing certain therapies and surgical procedures in this age group are compelling reasons to increase available evidence.
We describe cases of HS in patients older than 65 years at the initial visit, seen in a HS monographic consultation from October 2021 through May 2023. All patients signed their informed consent form prior to being included in the HS Registry of our center, which has been approved by the Balearic Islands ethics committee. Relevant comorbidities, time to first visit, clinical characteristics, phenotype according to Martorell et al.,5 lesion count to calculate the International Hidradenitis Suppurativa Severity Score System (IHS4), quantification of pain, pruritus, and the Dermatology Life Quality Index (DLQI) were recorded. Ultrasound was used to count the lesions.
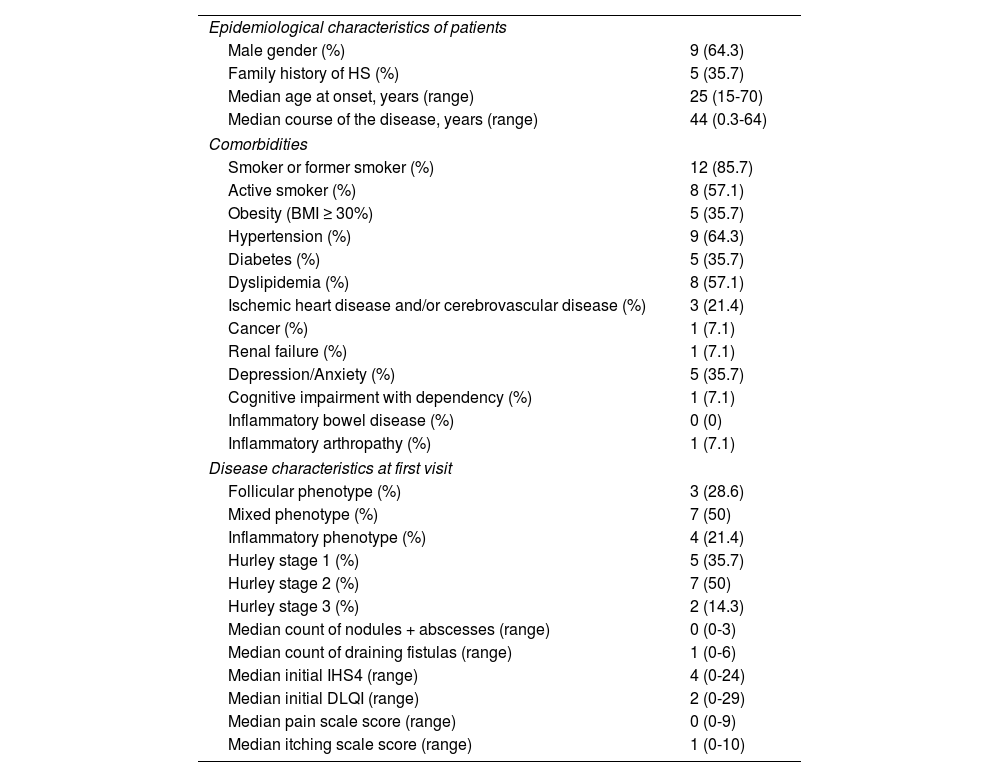
We included a total of 14 patients, which amounts to 4.8% of the all patients included in the HS Registry during this time (Table 1). The median follow-up time was 77 days (range, 0-466 days). Antibiotics were prescribed in 2 patients, metformin in 1, biologics in 2 (adalimumab in one patient and secukinumab in the other), retinoids in 1 patient, surgery in 3, and photodynamic therapy in 2 patients (external irradiation in one patient and intralesional irradiation in the other). One patient with an active lung neoplasm benefited from the insertion of a loose seton in a perianal fistula without need for further treatment for symptom control.
Clinical characteristics of treated patients > 65 years (n = 14).
| Epidemiological characteristics of patients | |
| Male gender (%) | 9 (64.3) |
| Family history of HS (%) | 5 (35.7) |
| Median age at onset, years (range) | 25 (15-70) |
| Median course of the disease, years (range) | 44 (0.3-64) |
| Comorbidities | |
| Smoker or former smoker (%) | 12 (85.7) |
| Active smoker (%) | 8 (57.1) |
| Obesity (BMI ≥ 30%) | 5 (35.7) |
| Hypertension (%) | 9 (64.3) |
| Diabetes (%) | 5 (35.7) |
| Dyslipidemia (%) | 8 (57.1) |
| Ischemic heart disease and/or cerebrovascular disease (%) | 3 (21.4) |
| Cancer (%) | 1 (7.1) |
| Renal failure (%) | 1 (7.1) |
| Depression/Anxiety (%) | 5 (35.7) |
| Cognitive impairment with dependency (%) | 1 (7.1) |
| Inflammatory bowel disease (%) | 0 (0) |
| Inflammatory arthropathy (%) | 1 (7.1) |
| Disease characteristics at first visit | |
| Follicular phenotype (%) | 3 (28.6) |
| Mixed phenotype (%) | 7 (50) |
| Inflammatory phenotype (%) | 4 (21.4) |
| Hurley stage 1 (%) | 5 (35.7) |
| Hurley stage 2 (%) | 7 (50) |
| Hurley stage 3 (%) | 2 (14.3) |
| Median count of nodules + abscesses (range) | 0 (0-3) |
| Median count of draining fistulas (range) | 1 (0-6) |
| Median initial IHS4 (range) | 4 (0-24) |
| Median initial DLQI (range) | 2 (0-29) |
| Median pain scale score (range) | 0 (0-9) |
| Median itching scale score (range) | 1 (0-10) |
There are few publications on HS in individuals older than 65 years old. In a recent publication, Blum et al. describe a cohort of 26 patients older than 65 years, which amounts to just 2.3% of all patients treated for HS.6
Same as in our series, a high prevalence of diseases in the metabolic and cardiovascular sphere—cancer included—significantly affect management and morbidity. Compared with the under 65 age group, there is a higher proportion of male patients and a greater presence of obesity and smoking.6 These comorbidities have been described as predictors of disease persistence in retrospective studies,7,8 which reinforces the importance of addressing them in consultation.
The proportion of patients with Hurley stages 2 and 3 in our series reaches two-thirds. However, only 2 patients (14.2%) had initiated treatment with a biologic drug. This could be due to several factors, such as the higher presence of comorbidities or the relatively low inflammatory burden, as evidenced by the low level of pain and relatively low IHS4 compared with Hurley stage. The presence of more draining fistulas (median 1, range 0 to 6), and a low count of inflammatory lesions such as nodules and abscesses (median 0, range 0 to 3) should favor the use of lesion-directed treatments such as surgery or photodynamic therapy. The latter, in various modalities, has demonstrated its utility in local HS control.9 Another technique directed at the lesion, interesting for its simplicity and effectiveness, is the use of setons, which has been described in HS with good symptom control.10
In conclusion, patients older than 65 years seen for the first time with an HS diagnosis represent a low proportion of the total (2.3% up to 5% overall). They have a high frequency of Hurley stages 2 and 3, which underscores the need for making all therapeutic options available, although some comorbidities limit access to biologic therapies or extensive surgery. Lesion-directed therapies may be an appropriate and sufficient alternative for symptom control.
Conflicts of interestJ. Garcias-Ladaria declares having participated as principal investigator in clinical trials for Acelyrin, having received financial support for attending congresses from Alfasigma, Isdin, Cantabria Labs, Janssen, Almirall, Novartis, and UCB, and having received honoraria from Novartis as an advisor and from Novartis and Leo-Pharma as a guest speaker.
Ines Gracia-Darder declares having given lectures at events held by UCB, Novartis, Sanofi, Almirall, Janssen, Isdin, Loreal, Pierre Fabre, having participated as a researcher in trials sponsored by Almirall, Leo-Pharma, Acelyrin, and Pierre Fabre, and having received support to attend congresses from Almirall, Janssen, Leo-Pharma, Lilly, Novartis, Pierre Fabre, Sanofi, UCB, Amgen, Isdin, Cantabria Labs, Rilastil, and Loreal.
Juan Jerónimo González Malmierca declared no conflicts of interest whatsoever.
Ana Martín-Santiago declares having collaborated as a consultant for Abbvie, Amryt, Leo-Pharma, Pfizer, and Sanofi, having given lectures at events held by Abbvie, Amgen, Leo, Leti, Novartis, Pfizer, Sanofi, and having signed research agreements with Almirall, Amgen, Lilly, Leo-Pharma, Novartis, Pierre Fabre, and Sanofi, and having received support to attend congresses from Abbvie, Almirall, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Pierre Fabre, Sanofi, UCB, and Viatrix.