Accurate information on the incidence of melanoma by stage and a better understanding of transition between stages are important for determining the burden of disease and assessing the impact of new adjuvant therapies on recurrence and survival. The aim of this study was to estimate the incidence rates of the various stages of melanoma in Spain and the number of patients with stage III disease who are eligible for adjuvant systemic therapies.
Materials and methodWe built an epidemiological model using prospectively collected data from patients diagnosed with de novo or recurrent melanoma between 2012 and 2016 in the melanoma units of 4 public hospitals.
ResultsThe estimated crude incidence rates for stage I and II melanoma were 7 and 2.9 cases per 100 000 person-years, respectively. The corresponding rates for stage III and IV melanoma were 1.9 and 1.3 cases per 100 000 person-years; 25.8% of patients with stage III melanoma were stage IIIA, 47% were stage IIIB, and 27.3% were stage IIIC. The respective estimated incidence rates for recurrent stage III and IV melanoma were 1.1 and 0.9 cases per 100 000 person-years. Overall, 54% of patients with recurrent stage III melanoma had progressed from stage I or II; the other cases corresponded to changes in substage. Of the patients with stage III melanoma, 85% of those with a de novo diagnosis and 80% of those who had relapsed had resectable disease, meaning they were eligible for adjuvant therapy; 47% of these patients had a BRAF mutation.
ConclusionsThe above estimates could have a major impact on health care resource planning. Assessing the number of patients with melanoma who are eligible for adjuvant therapies in melanoma could help decision-makers and clinicians anticipate future needs for the management of this disease.
Para estimar la carga real del melanoma y el impacto de las nuevas terapias adyuvantes sobre las recaídas y la supervivencia, se precisa conocer con mayor exactitud la incidencia por estadios y analizar la transición entre ellos. Este estudio pretende estimar dicha incidencia y determinar el número de pacientes en estadio III que podrían beneficiarse del tratamiento sistémico adyuvante en España.
Materiales y métodoSe elaboró un modelo epidemiológico basado en datos de pacientes diagnosticados de melanoma o en recaída, recogidos prospectivamente durante 2012-2016 por cuatro unidades de melanoma de centros sanitarios públicos.
ResultadosLas tasas brutas de incidencia estimadas para estadios I y II se situaron en 7 y 2,9 casos por 100.000 personas-año, respectivamente. Para estadio III se estimó en 1,9 (25,8% en IIIA, 47% en IIIB, y 27,3% en IIIC), siendo la de estadio IV de 1,3. La tasa de recaídas en estadio III se estimó en 1,1, siendo para estadio IV de 0,9. El 54% de recaídas a estadio III procedían de estadios I/II, mientras que el resto progresaban desde subestadios III. En estadio III, un 85% de nuevos diagnósticos y un 80% de recaídas fueron resecables, por tanto, candidatos a adyuvancia, de los cuales el 47% presentaba mutación en BRAF.
ConclusionesEstas estimaciones podrían tener un impacto importante en la planificación de los recursos sanitarios. La proyección en el número de potenciales candidatos a adyuvancia puede ayudar a decisores y clínicos a anticiparse a futuras necesidades en el manejo del melanoma.
Cutaneous melanoma accounts for approximately 4% of skin cancers worldwide; it has one of the highest mutational burdens1 and is responsible for 80% of all skin cancer deaths.2
The crude incidence of cutaneous melanoma in Spain rose from 8.8 cases per 100 000 person-years in 20153 to an estimated 13.1 cases in 2020.4 In Catalonia, the age-standardized incidence in 2017 was higher than in other parts of Spain, with a rate of 13.8 cases per 100 000 population.5 The above figures, however, might be underestimated as they are from registries covering about 30% of the Spanish population.6 They also do not provide any information on stage-specific incidence or transitions between stages in patients with recurrent disease. This makes it difficult to accurately determine the number of newly diagnosed cases within each stage.
Tumor stage, one of the main prognostic factors in melanoma, is established using the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.7 There is evidence of significantly reduced survival in advanced melanoma; patients with stage IV disease, for example, have a 5-year survival rate of just 9% to 28% compared with 95% to 100% for those with stage I disease.8 Risk of recurrence at each stage must also be considered when analyzing prognosis and is highest in patients with advanced disease.9BRAF mutations, present in about 50% of patients with melanoma,10 might be associated with progression. Together with number of positive lymph nodes, their presence has been identified as an independent prognostic factor significantly associated with survival and recurrence in resected stage IIIB/C melanoma.11 Patients with a BRAF mutation have lower disease-specific survival and a higher risk of recurrence and mortality.11,12
Clinical guidelines recommend adjuvant systemic therapy as a means of reducing recurrence and mortality risk in patients with resected stage III or IV melanoma.13,14 A real-life study of patients with stage III melanoma showed that adjuvant interferon α-2b therapy following surgical excision was associated with significantly longer recurrence-free survival than a watch and wait strategy.15 Data from clinical trials have also shown better recurrence-free survival outcomes in patients treated with new adjuvant therapies than those observed to date with interferon.16 By contrast, significantly lower disease-specific survival rates have been reported in patients with more advanced substages of stage III melanoma not treated with adjuvant therapy.17
Information on stage- and substage-specific melanoma incidence would help analyze transitions to more advanced stages, providing useful prognostic information as well as insights into the impact of adjuvant therapies on disease progression. Information of this nature, however, is scarce in Spain. A better understanding of candidates for adjuvant therapy would help determine the true burden of melanoma in our setting and its impact on health care resource utilization and costs.
The aim of this study was to estimate stage-specific incidence of melanoma in Spain and determine the number of patients with stage III disease who might benefit from adjuvant therapy. To do this, we built an epidemiological model built using real-life data collected before the introduction of new adjuvant therapies.
Material and MethodsWe built an epidemiological model using data collected prospectively over a 5-year period by melanoma units at 4 public hospitals that are referral centers for the treatment and management of melanoma: Instituto Valenciano de Oncología (IVO), Hospital Universitario Virgen Macarena (HUVM), Hospital Clínic de Barcelona (HCB), and Hospital Universitario 12 de Octubre (HU12O). These hospitals, located respectively in Valencia, Sevilla, Barcelona, and Madrid, serve a population of approximately 2 million inhabitants.
Data SourcesWe compiled the following information using data recorded by the melanoma units on patients diagnosed with de novo or recurrent melanoma between 2012 and 2016: incidence of de nova melanoma by stage (I/II/III/IV) and substage (IIIA/IIIB/IIIC) according to the seventh edition of the AJCC Cancer Staging Manual18; incidence of recurrent stage III and IV melanoma and previous stages; percentage of patients with resectable de nova melanoma by stage; percentage of patients with resectable recurrent stage III or IV melanoma; number of patients with de novo and recurrent melanoma eligible for adjuvant therapy; and number of patients with a BRAF mutation eligible for adjuvant therapy. All patients with stage III melanoma are routinely screened for BRAF mutations at the 4 hospitals.
The model was built using data available from each unit. The data were analyzed in aggregate and no information was collected on clinical outcomes, follow-up, or treatment.
Epidemiological ModelThe epidemiological model was built using Spanish population statistics from 2020, reports in the scientific literature, and real-life data collected by the 4 melanoma units. We entered data on stage-specific melanoma incidence and transitions between stages, distinguishing between new diagnoses and recurrences (Table 1). Since the model was built with data from different autonomous communities of Spain, stage-specific incidence rates for de novo melanoma were estimated using figures published in 2020 by the Spanish Network of Cancer Registries (REDECAN) based on crude incidence rates for melanoma provided by national cancer registries.4
Evidence from Clinical Practice and The Scientific Literature for Model Estimations.
| Clinical practice data | Literature data |
|---|---|
| 1) Estimated incidence of stage-specific de novo melanoma in Spain | |
| Incidence and mean percentage of patients with newly diagnosed melanoma by stage (including substages IIIA, IIIB, and IIIC)18 | Crude national incidence of melanoma in Spain in 2020 (13.1 cases per 100 000 person-years), based on data from the REDECAN Spanish Network of Cancer Registries4Spanish population in 2020 according to National Statistics Institute: 47 329 981 inhabitants19 |
| 2) Estimated number of patients with stage III and IV recurrences in Spain | |
| Incidence and percentage of patients with stage III or IV recurrences; percentage of patients who progress to stage III or IV from other stages | Catchment population: 500 000 inhabitants for each hospital29–31 Spanish population in 2020 according to National Statistics Institute19 |
| 3) Estimated number of patients with resectable stage III melanoma who might benefit from adjuvant therapy in Spain | |
| Mean annual number of patients with resectable recurrent stage III melanomaMean annual number of patients with resectable de novo stage III melanoma | Spanish population in 2020 according to National Statistics Institute19 |
| 4) Estimated number of patients in Spain with de novo or recurrent stage III melanoma and aBRAFV600 mutation who could benefit from adjuvant therapy | |
| Mean percentage of candidates with a BRAF V600 mutation eligible for adjuvant therapy | – |
As the study hospitals were all referral centers for the diagnosis and treatment of melanoma, the rates for resectable stage III melanoma might be higher than the national average. To account for this potential overestimation, we applied a correction factor agreed on by the expert team based on their experience. It was assumed that the rate of resectable stage III melanomas was probably 10% higher than the national average and a correction factor of 10% was therefore applied to rates reported for de novo and recurrent melanoma.
The means estimated in the model were calculated with their standard deviation. The data from the registries were recorded and analyzed in Microsoft Excel.
ResultsDescription of Data CollectedWe collected data on 1795 patients with de novo stage I–IV melanoma (IVO: 279, HUVM: 286, HCB: 1024, and HU12O: 206) and 402 patients with recurrent stage III or IV melanoma (IVO: 86, HUVM: 27, HCB: 252, and HU12O: 37).
Stage-Specific Incidence of De Novo MelanomaThe incidence rates for newly diagnosed melanoma are shown according to stage in Table 2; 53.5% of cases were stage I, 22.4% stage II, 14.2% stage III, and 9.9% stage IV.
Stage-Specific Incidence of De Novo Melanoma Recorded by Melanoma Units for the Period 2012–2016.
| IVO | HVM | HCB | HU12O | Mean % (SD)c | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD)b | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Stagea | |||||||||
| Stage I | 30.0 (6.1) | 53.8 | 39.4 (4.3) | 68.4 | 98.4 (11.5) | 48.1 | 24.2 (2.4) | 59.0 | 53.5 (8.6) |
| Stage II | 11.2 (5.2) | 20.1 | 10.0 (2.2) | 17.4 | 46.0 (6.9) | 22.5 | 13.2 (3.0) | 32.2 | 22.4 (6.4) |
| Stage III | 13.2 (4.1) | 23.7 [100] | 7.2 (0.8) | 12.5 | 28.0 (5.0) | 13.7 | 2.6 (1.7) | 6.3 | 14.2 (7.2) |
| IIIA | 3.4 (1.9) | 6.1 [25.8] | – | – | – | – | – | – | – |
| IIIB | 6.2 (3.6) | 11.1 [47] | – | – | – | – | – | – | – |
| IIIC | 3.6 (1.3) | 6.5 [27.3] | – | – | – | – | – | – | – |
| Stage IV | 1.4 (0.5) | 2.5 | 1.0 (0.9) | 1.7 | 32.0 (7.4) | 15.7 | 1.0 (0.4) | 2.4 | 9.9 (6.8) |
| Total annual incidence | 55.8 | 100 | 57.6 | 100 | 204.4 | 100 | 41.0 | 100 | 100 |
Abbreviations: AJCC, American Joint Committee on Cancer; HCB, Hospital Clínic de Barcelona (Barcelona); HU12O, Hospital Universitario 12 de Octubre (Madrid); HVM, Hospital Virgen Macarena (Sevilla); IVO, Instituto Valenciano de Oncología (Valencia).
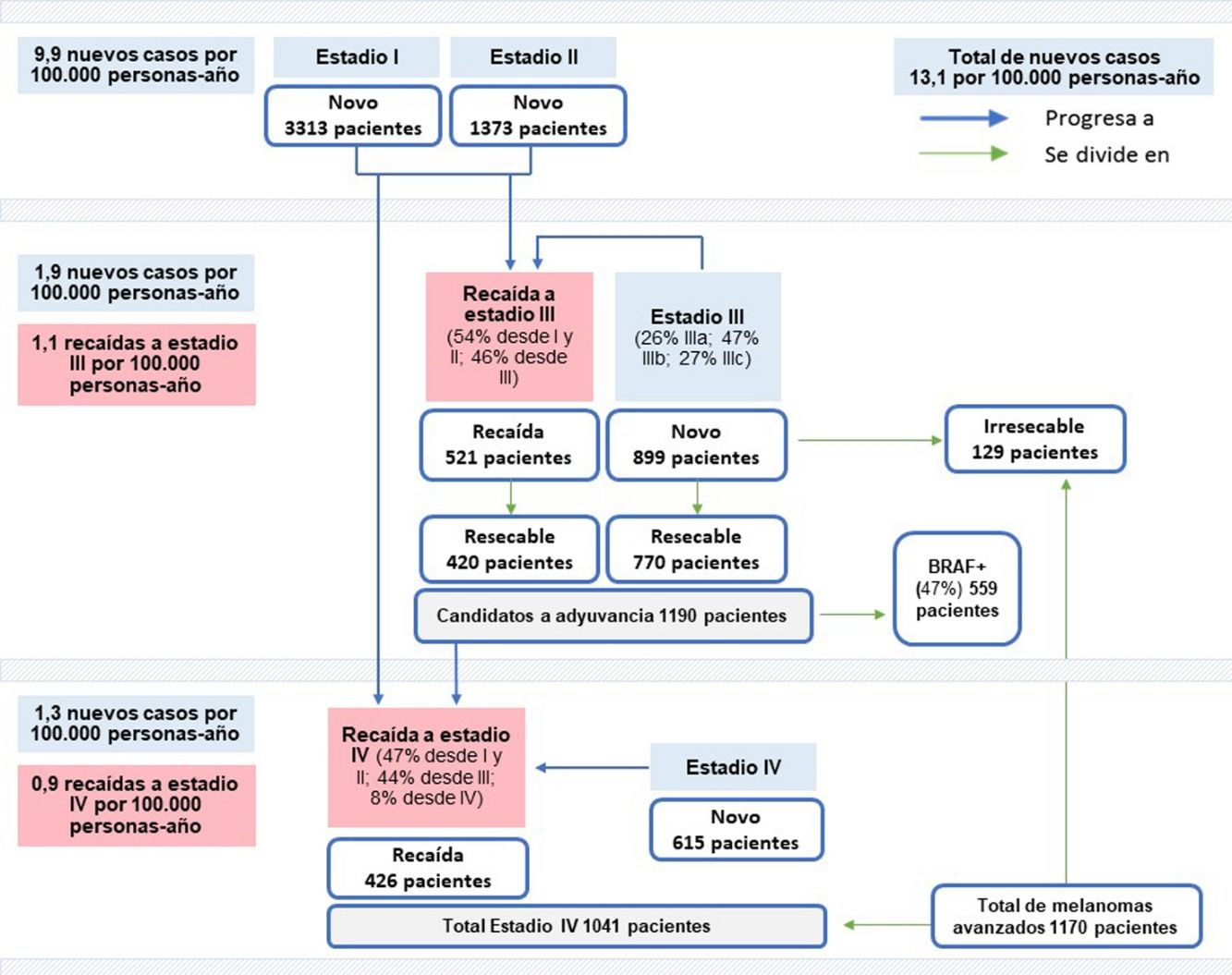
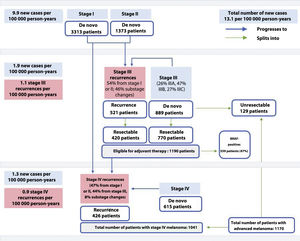
Based on the crude incidence rates reported for melanoma in Spain in 20204 and the mean percentages of patients in each stage, we estimated a crude incidence rate of 7 cases per 100 000 person-years for stage I, 2.9 cases per 100 000 person-years for stage II, 1.9 cases per 100 000 person-years for stage III, and 1.3 cases per 100 000 person-years for stage IV. On extrapolating these figures to the census data for 2020,19 we calculated that 6200 new cases of melanoma would have been diagnosed in Spain that year; of these, 3313 would have been stage I, 1373 stage II, 899 stage III, and 615 stage IV (Fig. 1).
Newly diagnosed melanoma cases: 6200 cases overall (13.1 per 100 000 person-years×47 329 981 inhabitants), 3313 stage I cases (7 per 100 000 person-years×47 329 981 inhabitants), 1373 stage II cases (2.9 per 100 000 person-years×47 329 981 inhabitants), 899 stage III cases (1.9 per 100 000 person-years 47 329 981 inhabitants), and 615 stage IV cases (1.3 per 100 000 person-years×47 329 981 inhabitants). Recurrent melanoma cases: 521 stage III cases (1.1 per 100 000 person-years×47,329,981 inhabitants) and 426 stage IV cases (0.9 per 100 000 person-years×47,329,981 inhabitants). Candidates for resection: 420 patients with recurrent melanoma (521×80.7%) and 770 with de novo melanoma (899×85.7%); overall, 1190 patients (420+770) with resectable disease and 129 (899−770) with unresectable de novo disease. Patients with BRAF mutations eligible for adjuvant therapy: 559 (1190 patients×47%). Total number of patients with stage IV melanoma: 1041 (426+615). Total number of patients with advanced melanoma: 1170 (1041+129).
Based on the substage-specific incidence rates for stage III melanoma (data from IVO) and the number of newly diagnosed stage III melanomas estimated for 2020 (899), we calculated that there would have been 232 stage IIIA cases, 422 stage IIIB cases, and 245 substage IIIC cases (Table 2).
Incidence of Recurrent Melanoma and Previous StagesThe incidence rates for recurrent stage III and IV melanoma are shown in Table 3. According to our estimations, stage III recurrences were more common than stage IV recurrences (54.9% vs. 45.1%).
Stage-Specific Incidence of Recurrent Stage III and IV Melanoma Recorded by Melanoma Units for the Period 2012–2016.
| IVO | HVM | HCBb | HU12O | Mean % (SD)c | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD)a | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Stage | |||||||||
| Stage III | 10.0 (5.4) | 58.1 | 2.4 (0.5) | 44.4 | – | – | 4.6 (1.1) | 62.2 | 54.9 (9.3) |
| Stage IV | 7.2 (1.9) | 41.9 | 3.0 (1.6) | 55.6 | – | – | 2.8 (0.8) | 37.8 | 45.1 (9.3) |
| Total annual incidence | 17.2 | 100 | 5.4 | 100 | – | – | 7.4 | 100 | 100 |
Abbreviations: HCB, Hospital Clínic de Barcelona (Barcelona); HU12O, Hospital Universitario 12 de Octubre (Madrid); HVM, Hospital Virgen Macarena (Sevilla); IVO, Instituto Valenciano de Oncología (Valencia).
Based on the incidence rates for recurrent stage III melanoma by hospital and the hospitals’ catchment populations, we estimated a crude incidence rate of 1.1 recurrences per 100 000 person-years (2 at IVO, 0.5 at HUVM, and 0.9 at HU120).
The crude incidence rate estimated for stage IV recurrences (Table 3) was 0.9 cases per 100 000 person-years.
On extrapolating the data to the general Spanish population in 2020, it was estimated that there would have been 521 cases of locoregional (stage III) recurrence and 426 cases of distant (stage IV) recurrence (Fig. 1).
Based on the total number of recurrent stage III and IV melanomas, we calculated the distribution of cases according to previous stage (Table 4). Overall, 54% of patients with recurrent stage III melanoma would have progressed from stage I or II, while 46% would have progressed from another substage (lymph node spread only). The corresponding number of patients based on the data for the Spanish population in 2020 would have been 281 and 240, respectively.
Finally, we calculated that 47.2% of patients with recurrent stage IV melanoma would have progressed from stage I or II, while 44.4% would have progressed from stage III.
Patients with Stage III Melanoma Eligible for Adjuvant TherapyOverall, 100% of newly diagnosed stage I and II melanomas and 85.7% of newly diagnosed stage III melanomas were considered resectable (Table 5). The majority of stage IV melanomas, by contrast, were considered unresectable.
Percentage of Patients with Resectable Melanoma by Stage at Each Hospital.
| IVO | HVM | HCB | HU12O | Mean percentage | |
|---|---|---|---|---|---|
| De novo resectable melanoma | |||||
| Stage I | 100% | – | 100% | 100% | 100% |
| Stage II | 100% | – | 100% | 100% | 100% |
| Stage III | 100% | 97% | 90% | – | 95.7% |
| Stage IV | 0% | – | 10% | – | 5% |
| Recurrent resectable melanoma | |||||
| Stage III | 91.4% | 90% | – | – | 90.7% |
| Stage IV | 26.4% | 29% | – | – | 27.7% |
Abbreviations: HCB, Hospital Clínic de Barcelona (Barcelona); HU12O, Hospital Universitario 12 de Octubre (Madrid); HVM, Hospital Virgen Macarena (Sevilla); IVO, Instituto Valenciano de Oncología (Valencia).
On applying the 10% correction factor to address the possible overestimation of resectable stage III melanomas, it was considered that 85.7% of all newly diagnosed melanomas in the general Spanish population would be resectable. In other words, 770 of the 899 patients with de novo stage III melanoma would have been candidates for adjuvant therapy after resection (Fig. 1).
In the case of recurrent stage III melanoma, 90.7% of tumors were considered resectable. On applying the 10% correction factor, it was calculated that 420 of the 521 patients with recurrent stage III disease would have been candidates for resection.
On extrapolating these data to the Spanish population, 1190 patients (770+420) with stage III melanoma would have been eligible for adjuvant therapy in 2020 (Fig. 1).
Patients with a BRAF V600 Mutation Eligible for Adjuvant TherapyWe estimated that 47% of patients with resectable de novo and recurrent stage III melanoma would have had a BRAF mutation (Table 6).
Mean Number of Candidates for Adjuvant Therapy and Mean Number of Patients with a BRAF Mutation at Each Hospital Between 2012 and 2016.
| IVO | HVM | HCB | HU12O | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Eligible for adjuvant therapy | 12.2 (5.5) | 9.2 (1.3) | 29.2 (5.3) | – |
| BRAF-positive patients eligible for adjuvant therapy | 6.8 (3.6) | 3.8 (1.1) | 12.8 (2.3) | – |
| Percentage | 55.7% | 41.3% | 43.8% | – |
Abbreviations: AJCC, American Joint Committee on Cancer; HCB, Hospital Clínic de Barcelona (Barcelona); HU12O, Hospital Universitario 12 de Octubre (Madrid); HVM, Hospital Virgen Macarena (Sevilla); IVO, Instituto Valenciano de Oncología (Valencia).
Accordingly, 559 BRAF-positive patients would have been candidates for adjuvant therapy (Fig. 1).
DiscussionMelanoma patients at high risk of recurrence are candidates for adjuvant therapy. In this study, we estimated how many patients with stage III melanoma in Spain might be eligible for this treatment.
Our calculations indicate that 14% of patients have stage III melanoma at diagnosis. This rate is higher than that estimated in an earlier Spanish study using population-based data from the national melanoma registry for the period 1997–2011 (9.9%)20 and also higher than that reported in the United States for the period 2008–2017 (8.5%).21 The differences may be due to the fact that we used data from referral hospitals for the treatment of melanoma located in large cities that diagnose more cases of advanced melanoma than the national average. The 10% incidence rate observed for stage IV melanoma is also higher than in previous reports.20,21
While our estimates may not fully reflect melanoma incidence in Spain, they show that approximately 25% of patients treated at referral hospitals have advanced melanoma at diagnosis and are consequently at an increased risk of recurrence. According to data from a study conducted prior to the introduction of new therapies for advanced melanoma, 5-year recurrence-free rates among patients with stage IIIA, IIIB, and IIIC melanoma ranged from 10% to 60%, with stage IIIC patients showing the highest risk of relapsing.22 A 2018 study of relapse patterns in melanoma reported that 50% of distant metastases occurred in patients with stage III disease.9 According to our findings, approximately 50% of recurrent stage III cases corresponded to substage changes; in the case of recurrent stage IV melanoma, 44% of patients had progressed from stage III.
Other European studies conducted using clinical practice data have reported higher regional recurrence rates in patients with primary stage I or II melanoma23 and higher distant metastasis rates in those with primary stage III melanoma.9,24 In our study, the incidence rates for recurrent stage III and IV melanoma were similar and the patients had progressed from similar stages; a previous diagnosis of stage I or II melanoma was also slightly more common than one of stage III melanoma in both groups, even though stage III disease has a higher risk of distant metastasis.
Our findings highlight the need for new therapies in patients with advanced melanoma. Recent studies of patients who have undergone resection for stage III melanoma have shown that new treatments, including immunotherapy and targeted BRAF inhibition, reduce the risk of recurrence,25 mainly locoregional,26 with an acceptable safety profile.10 Based on Spanish census data, we estimated that 1190 patients would have been eligible for adjuvant therapy in our country in 2020. Our results also indicate that nearly 50% of patients with resectable stage III melanoma have a BRAF mutation and could potentially benefit from targeted adjuvant therapy.
Despite the advances made in melanoma treatment, not all hospitals or regions have the same access to new therapies, and access may also be limited by barriers to the implementation of biomarkers for treatment selection.27 Differences may largely be due to the high costs associated with new therapies. Nonetheless, it is important to determine whether the incorporation of these treatments into clinical practice can reduce recurrence rates and health care costs. Stage-specific information on melanoma incidence can be used to evaluate the economic impact of these therapies and help optimize the allocation of health care resources.
Our study has some limitations. First, the use of data from referral hospitals for the treatment and management of melanoma may have led to an overestimation of incidence, although we did apply a correction factor to bring the rates closer to the national average. While the crude incidence rate of 13.1 cases per 100 000 person-years used in our model is higher than that described by Tejera-Vaquerizo et al.3 in a meta-analysis published in 2016, it is similar to figures published by the REDECAN Spanish Network of Cancer Registries in 2015.4 Podlipnik et al.5 also reported a lower rate in a more recent study of melanoma incidence in Catalonia. We, however, decided to use the national incidence rate reported by REDECAN for 2020. Second, our epidemiological model was built before the introduction of new adjuvant therapies, when the seventh edition of the AJCC Staging Manual was still in use. The eighth edition introduced significant changes to the stage III staging system, and in some patients these modifications led to restaging.26 We do not believe, however, that the management of melanoma in Spain has changed significantly in this time as no novel adjuvants have been introduced. Because new adjuvant therapies are likely to reduce the risk of recurrence in patients with stage III melanoma, we suggest replicating this model after their introduction. Third, because we did not have specific data on tumor burden, all patients with stage IIIA disease were considered to be eligible for adjuvant therapy, even though this option is not recommended for patients with a largest lymph node size of less than 1mm. Our figures therefore may be overestimated. Finally, because we did not analyze the age of our patients, or other descriptive characteristics of the population, we were unable to check whether they are representative of the national population. Because older age is associated with a higher risk of treatment rejection and the presence of comorbidities that might preclude the use of adjuvant therapies, the number of potential candidates for novel adjuvants could be lower in older populations. Nonetheless, because we analyzed data from 4 hospitals located in large cities in different parts of Spain, we believe that our population is as representative as possible.
Finally, it should be noted that previous projections of melanoma incidence have been based on registry data that did not distinguish between stages.28 The figures therefore might be underestimated. Our study took a new approach by calculating stage-specific incidence at a national level.
ConclusionsAdjuvant therapy is an important consideration in the management of patients with stage III melanoma due to their lower survival rates and increased risk of recurrence. The estimates provided in this study could have an important impact on health care resource planning. Being able to predict the number of potential candidates for adjuvant therapies could help decision-makers and clinicians anticipate future needs in the management of melanoma.
FundingNovartis Farmacéutica S.A. financed the publication of this article. The company had no role in the collection or processing of data.
Conflicts of InterestP. Ortiz-Romero has received consultancy fees from Kyowa, Takeda, 4SC, Helsinn, Recordati Rare Diseases, Innate Pharma, and Miragen.
S. Puig has received consultancy or speakers’ fees from and/or has participated in clinical trials sponsored by Abbvie, Almirall, Amgen, BMS, Biofrontera, Canfield, Cantabria, Fotofinder, GSK, ISDIN, La Roche Posay, Leo Pharma, MSD, MEDA, Novartis, Polychem, and Roche.
E. Martín-Sánchez and A. Martínez-Fernández are full-time employees at Novartis Farmacéutica S.A.
The rest of the authors declare no conflicts of interest.
The authors are grateful to Outcomes’10 for their scientific advice on data analysis and the preparation of this manuscript.