Chronic hand and/or foot eczema (CHFE) is a chronic skin disease that can have a significant impact on the patients’ quality of life.1 Currently, alitretinoin is the only systemic drug authorized for this indication in the European Union.2 In recent years, dupilumab, a human monoclonal antibody targeting interleukin-4 and interleukin-13, has proven its efficacy treating patients with atopic dermatitis (AD), and its various phenotypes or related disorders such as nodular prurigo or CHFE.
This study describes the real-world experience of dupilumab in patients with CHFE. We designed a multicenter, retrospective, observational study with adult patients diagnosed with CHFE on dupilumab, with or without AD lesions in other anatomical regions. Cases of relevant allergic sensitization responsible for the patient's chronic eczema were excluded. Treatment response was assessed objectively (partial improvement if a 75% improvement was achieved or total if lesions were completely gone) and through patient-reported outcomes (PRO) at each clinical visit, using the Visual Analog Scale (VAS) for itch and the Dermatology Life Quality Index (DLQI).
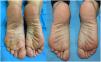
We enrolled a total of 11 patients (9 women [82%] and 2 men [18%]), with a median age of 40 years (range, 28-75). Their clinical-epidemiological characteristics are shown in Table 1. Five patients (45%) had a past medical history of AD onset in childhood (2) or adulthood (3). In 6 patients (54%) only the palms of their hands and/or the soles of their feet were compromised. Lesions in these locations had a mean 4-year history (range, 6 months to 20 years), and 2 patients exhibited the dyshidrotic eczema phenotype vs the remaining 9 who exhibited the hyperkeratotic phenotype. Five patients (45%) had been treated with oral retinoids, 3 of them (27%) with methotrexate, and 2 (18%) with cyclosporine. Two (18%) had received previous treatment with drugs approved for use in psoriasis such as apremilast, brodalumab, or ixekizumab. The median baseline DLQI was 16 (range, 13-28), and the median itch VAS was 9 (range, 8-10). The median follow-up was 68 weeks. All patients reported lesion improvement within the first 4 weeks of treatment, and 8 of them (72%) achieved complete lesion improvement within the first 4 months of therapy (Figures 1 and 2). The median DLQI on week 16 was 2 (range, 0-16), representing a 78.3% reduction vs baseline. Treatment was up-titrated every 10 days at the follow-up in 1 patient due to partial response, while another patient had to discontinue treatment on week 16 due to inefficacy. The remaining patients (80%) continued with the drug. One patient down-titrated every 21 days. No adverse events were reported.
Clinical-epidemiological characteristics of patients with chronic hand and/or foot eczema treated with dupilumab.
| Sex, age | Previous atopic history | History of palmar-plantar lesions | Distribution and lesion phenotype | Previous treatments | Baseline PROs | Response to dupilumab |
|---|---|---|---|---|---|---|
| Woman, 40 years | Childhood ADAllergic sensitization to nickel and cobalt | 10 years | Hyperkeratotic plantar eczema | Topical corticosteroids and calcineurin inhibitors, AntihistaminesOral corticosteroidsAlitretinoinAcitretin | DLQI 28Itch 10/10 | 75% improvement at 1 month, at 2 months itch reduced to 2/10 and DLQI to 7/30, escalated every 10 days. |
| Woman, 28 years | Nickel sensitization | 10 years | Hyperkeratotic eczema on 30% of both hands and 40% of both feet | Topical corticosteroids and calcineurin inhibitors, AntihistaminesAcitretinMethotrexateApremilastBrodalumab, Cyclosporine | DLQI 16Itch 9/10 | Whitened after 1 month of treatment |
| Man, 75 years | Allergic sensitization to fragrance mix I, thiomersal, and balsam of Peru | 10 years | Hyperkeratotic eczema on palms and soles | Topical corticosteroids and calcineurin inhibitors, AntihistaminesAlitretinoin | DLQI 13Itch 8/10 | Whitened after 2 months of treatment |
| Woman, 67 years | None | 20 years | Hyperkeratotic eczema on palms and soles, dyshidrotic eczema on hands | Topical corticosteroids and calcineurin inhibitors AntihistaminesAcitretin | DLQI 23Itch 9/10 | Slight improvement in fissures, discontinued at 16 weeks due to inefficacy |
| Woman, 33 years | Allergic asthma, sensitization to dust mites and pellitory Perennial rhinitis | 6 months | Dyshidrotic eczema on soles of feet and hands | Topical corticosteroids and calcineurin inhibitorsOral corticosteroids | DLQI 19Itch 10/10 | Whitened after 2 months of treatment (figure 1) |
| Woman, 36 years | Adult ADNickel sensitization | 2 years | Plantar hyperkeratotic eczema, atopic eczema lesions on trunk, legs, and arms | Topical corticosteroids and calcineurin inhibitors, AntihistaminesOral corticosteroids, Methotrexate | DLQI 15Itch 9/10 | 50% improvement at 1 month, completely whitened at 6 months, down-titration every 21 days (figure 2) |
| Woman, 32 years | Childhood AD | 2 years | Hyperkeratotic eczema on palms (and dorsum) and flexural | Topical corticosteroids and calcineurin inhibitorsOral corticosteroidsMethotrexate | Itch/sleep: 10/10 DLQI 15 | DLQI 1 on week 4DLQI 0 and EASI 0 on weeks 16, 52, and 104 |
| Woman, 56 years | Adult ADACD to rubber (nurse) | 3 years | Palmar hyperkeratotic eczema, Subacute eczema on back and flexures | Topical corticosteroids and calcineurin inhibitorsOral corticosteroidsCyclosporine | EASI 18BSA 33%DLQI 19, Itch/sleep: 10/8 | DLQI 0 and itch 2 on week 4DLQI 0 and itch 1 on week 16Whitened since week 16 |
| Man, 71 years | Adult ADACD to perfumes (isoeugenol, oil of cloves) | 7 years | Hyperkeratotic eczema on handsEczema on trunk and limbs | Topical corticosteroids and calcineurin inhibitorsOral corticosteroids | Itch/sleep: 8/6, DLQI 15EASI 21, BSA 25% | Itch 0 on week 4Whitened since week 16 with DLQI 2 |
| Woman, 39 years | None | 2 years | Dyshidrotic eczema | Topical corticosteroids and calcineurin inhibitorsOral corticosteroids | DLQI 22Itch 10/10 | Itch 1 on week 4Whitened from week 16 with DLQI 0 |
| Woman, 53 years | None | 4 years | Hyperkeratotic eczema on hands and feet | Topical corticosteroids and calcipotriol, AcitretinIxekizumab | DLQI 16Itch 9/10 | 75% improvement at 1 month, no lesions at 2 months, itch 1/10 and DLQI 2/30 |
ACD, allergic contact dermatitis; AD, atopic dermatitis; BSA, body surface area; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; PRO, patient reported outcomes.
The efficacy of dupilumab in palmoplantar involvement has been reported in several case series. Osterhaven et al. presented a prospective, observational study of 47 patients, 74.5% of whom exhibited chronic fissured eczema and 25.5%, vesicular eczema treated with dupilumab, 60% of whom improved—75% compared to baseline—with no differences reported between both clinical subtypes. Additionally, improvement was observed within the first 4 weeks of treatment becoming substantial improvement within the first 16 weeks,3 which are data consistent with those presented in our study. In our patients, the median DLQI reduction was almost 80%, which is similar to what the Dutch series reported (70%). Voorberg et al. published the results of a prospective analysis of 72 patients with CHFE with or without AD treated with dupilumab and a 52-week follow-up, achieving a 75% improvement in the Hand Eczema Severity Index (HECSI-75) and HECSI-90 in 87.1% and 62.9%, respectively, with progressive clinical improvement from week 16 to week 52 and an improved quality of life.4 Finally, Lee et al. retrospectively compared the response to dupilumab between patients with AD and hand involvement (38/66) and those with AD only without hand involvement (28/66), and found no significant differences between both subgroups.5 Recently, Olesen et al. reported their experience with 19 patients with chronic hand eczema, and confirmed improvement in 73.7% of them.6 Of note in this latter study is the use of biologic drugs approved for psoriasis, as it is the case of 2 of the 11 patients from our series, which reveals the diagnostic challenge posed by hyperkeratotic hand and/or foot eczema without any other suggestive cutaneous lesions of AD or psoriasis.7
The experience reported has led to the phase 3 randomized double-blind LIBERTY-AD-HAFT trial NCT04417894 that is evaluating the safety and efficacy profile of dupilumab in adults and adolescents with CHFE. A total of 133 patients were randomized on a 1:1 ratio to receive dupilumab vs placebo, concluding that dupilumab improved the signs, symptoms, and quality of life of patients with hand and foot eczema measured by Investigator Global Assessment (IGA) 0/1, a > 4 point improvement in HECSI, itch VAS, or quality of life scale.8
Limitations of our analysis include its small sample size and absence of some data typical of a retrospective study, which would have given greater robustness to the results. On the other hand, The HECSI was not measured, nor were other PROs such as pain, stinging, or specific quality of life scales.
In conclusion, in our experience, patients with CHFE show both clinical and subjective improvement early after initiating treatment with dupilumab. Longer-term follow-ups of patients and more cases supporting the use of this drug for the treatment of these conditions are necessary.
Conflicts of interestNone declared.