Rosacea is a chronic disease negatively impacting the patients’ quality of life and mental health. The Rosacea Quality of Life (RosaQoL) scale could be a useful tool to monitor patients while on therapy vs rosacea, as it measures the impact on quality of life and helps individualize treatment to meet the patients’ needs. RosaQoL is a validated scale that can be completed within a few minutes.
Materials and methodsThe original scale was translated and back translated by 2 native translators, with input from an expert committee when necessary. This version was tested on 21 patients to ensure proper understanding. Psychometric characteristics and validity were determined using various measures (sensitivity and specificity via ROC curve and internal consistency via Cronbach's alpha). The correlation between RosaQoL and SF-12 scales was assessed using Pearson correlation coefficients.
ResultsA total of 531 participants responded to the scale (481 with rosacea and 50 controls). The scale demonstrated excellent sensitivity and specificity (ROC curve, 0.96; 95%CI, 0.92-0.99) and high internal consistency (Cronbach's alpha, 0.96). RosaQoL correlated with SF-12. A higher score on the RosaQoL scale was associated with worse quality of life in all dimensions of the SF-12 scale.
ConclusionsThe Spanish version of the RosaQoL scale exhibits psychometric characteristics, which are similar to the original scale. Also, the RosaQoL scale is useful to assess the quality of life of patients with rosacea.
La rosácea es una enfermedad crónica que afecta negativamente a la calidad de vida y la salud mental de los pacientes. La escala Rosacea Quality of Life (RosaQoL) podría ser una herramienta útil para seguir a los pacientes durante el tratamiento de la rosácea, ya que mide el impacto en la calidad de vida y ayuda a adaptar el tratamiento a las necesidades del paciente. Es una escala validada, que se cumplimenta en pocos minutos.
Materiales y métodosSe realizó la traducción y retrotraducción de la escala original, por parte de dos traductores nativos, con el consejo de un comité de expertos cuando fue necesario. Esta versión fue testada en 21 pacientes para comprobar la correcta comprensión. Las características psicométricas y su validez se determinaron utilizando varias medidas (sensibilidad y especificidad mediante curva ROC y consistencia interna por alfa de Cronbach). La correlación entre escalas RosaQoL y SF-12 se realizó mediante coeficientes de correlación de Pearson.
ResultadosUn total de 531 participantes respondieron a la escala (481 con rosácea y 50 controles). La escala presentó una excelente sensibilidad y especificidad (curva ROC: 0,96; IC 95%: 0,92-0,99) y una elevada correlación interna (alfa de Cronbach: 0,96). La escala RosaQoL se correlacionó con la SF-12. Una mayor puntuación en la escala RosaQoL se asoció con una peor calidad de vida en todas las dimensiones de la escala SF-12.
ConclusionesLa versión española de la escala RosaQoL presenta características psicométricas similares a la escala original, y es útil para evaluar la calidad de vida en los pacientes con rosácea.
Rosacea is a chronic skin disease that can also damage the eyes. It typically occurs in middle-aged and older adults with fair skin, especially women. Symptoms include redness, papules, and pustules, and in some patients, thickening and fibrosis of the skin, known as phyma.1
Rosacea negatively impacts the patients’ quality of life and mental health due to the presence of erythema and facial lesions that severely alter self-image and self-esteem.2,3
Health-related quality of life (HRQoL) measurement tools aim to objectively evaluate how a certain disease affects the life of an individual. These questionnaires offer insights into the impact of the disease on overall quality of life or specific areas, such as functionality, emotions, etc.4.
HRQoL measurement can be done in 2 ways: using generic instruments that assess quality of life globally, such as the SF-12 questionnaire,5 and specific instruments to deal with problems associated with particular disorders, patient types, or functional areas,6,7 such as the Dermatology Life Quality Index [DLQI]8 or the SKINDEX-29,9 both validated in Spanish language).
The SF-12 scale, widely used in research, has been associated with specific scales such as the DLQI in atopic dermatitis.10 However, these generic scales may not adequately capture the impact of certain diseases like rosacea. The Rosacea Quality of Life (RosaQoL) scale—which is specific to rosacea—is available in English and measures the impact of its symptoms on quality of life, being more precise than generalist scales.11 Having a validated tool in Spanish will allow for assessing the impact on the patient's quality of life, and individualizing treatment effectively.4,5
The objectives of this study are: 1) to validate and culturally adapt the RosaQoL scale into Spanish and 2) to analyze the correlation between the RosaQoL scale and the SF-12 scale.
Materials and methodsStudy designThe study was conducted from August through December 2021 after being approved by Hospital Universitario Sagrat Cor de Barcelona (Barcelona, Spain) Research and Ethics Committee, and consisted of the translation and cultural adaptation of the RosaQoL scale into Spanish, following the 5 stages indicated in international literature12–14: 1) direct translation of the original questionnaire into Spanish by, at least, 2 bilingual translators independently; 2) synthesis and resolution of any discrepancies in the translations; 3) back-translation into the original language by, at least, 2 independent translators who were blinded to the original version; 4) review by an expert committee, consisting of the 3 study coordinators recognized as Key Opinion Leaders in rosacea, to ensure semantic, idiomatic, cultural, and conceptual equivalence; and 5) pilot testing of the translated questionnaire with 21 subjects (7 healthy controls, 2 patients with rosacea, and 12 patients with various dermatological conditions), similar to the target population.
To make sure that the timing associated with response did not influence the result, a test-retest was conducted through which a group of 10 patients completed the scale yet again within 1-2 days of the initial visit. Additionally, to analyze discrimination between cases and controls, a group of 50 subjects without rosacea (controls) completed the scale too. At the end of this phase, a final version of the RosaQoL questionnaire was ready for validation.
In a 2nd phase, a total of 481 patients completed the RosaQoL and SF-12 scales for questionnaire validation purposes.
To assess sensitivity to change, the same patients completed both scales again 3 months later.
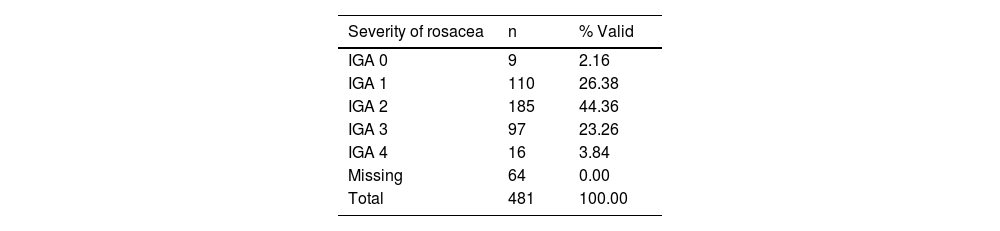
PatientsCultural adaptation to Spanish was performed with 531 adult participants attending 17 dermatology clinics in Spain. The sample consisted of 481 participants with varying severity of rosacea based on the Investigator Global Assessment (IGA) scale15 (cases) and 50 healthy participants (controls).
RosaQoL questionnaireThe RosaQoL questionnaire is available in English and measures the impact of the disorder and its symptoms on quality of life, being more precise than generalist scales as it is specific to rosacea-related quality of life.11
It is a self-administered tool developed from the SKINDEX-29 questionnaire consisting of 21 items categorized into 3 dimensions or domains: symptoms (7 items), functionality (3 items), and emotional state (11 items). Each item has a response scale with 5 possible options, being 0: never; 1: rarely; 2: sometimes; 3; often; and 4: always.11
SF-12 questionnaireThe SF-12 health questionnaire is available in Spanish and measures 8 different aspects of HRQoL.16,17
The SF-12 scale is a self-administered tool developed from the SF-36 scale consisting of 12 items categorized into 8 different dimensions or domains: physical function (2 items), physical role (2 items), bodily pain (1 item), general health (1 item), vitality (1 item), emotional role (2 items), social function (1 item), and mental health (2 items).16
IGA scaleThe IGA scale is an ordinal scale with 5 categories that evaluate the severity of lesions. Classification goes from 0 (no inflammatory lesions or erythema) up to 4 (intense erythema and/or numerous papules and pustules).15
Statistical analysisData analysis was performed using the SAS System version 9.4 statistical package (SAS Institute Inc., Cary, North Carolina, United States).
The questionnaire will be considered discriminative the closer the area under the ROC (Receiver Operating Characteristic) curve is to a value of 1.00.18 Both the sensitivity and specificity rates of the questionnaire were evaluated using Youden's criterion,19 and the internal consistency reliability of the questionnaire was measured using Cronbach's alpha coefficient.20 Following the criterion recommended by George and Mallery,21 a Cronbach's alpha coefficient ≥ 0.90 indicates excellent internal consistency. To evaluate test-retest reliability, the method proposed by Bland and Altman22 and the intraclass correlation coefficient proposed by Shrout and Fleiss23 were used. An intraclass correlation coefficient > 0.90 indicates high reliability of the questionnaire.24 Convergence analysis between the RosaQoL scale and the more general SF-12 scale was obtained using Pearson correlation coefficient (results between 0.4 and 0.7 were considered satisfactory).25
ResultsThe translated RosaQoL questionnaire (Table 1) was submitted to 531 participants with a mean age of 47 years (SD, 13.4 [95%CI, 45.8-48.1]), 71 of whom (n=369) were women and 29% (n=151), men. Among those with rosacea, the papulopustular type was the most common, representing 65.9% of the cases.
Items of the RosaQoL Questionnaire.
| How often have you identified yourself with any of these statements over the past 4 weeks? | |
|---|---|
| 1 | I worry that my rosacea could be severe |
| 2 | My rosacea burns or itches |
| 3 | I worry about scarring from my rosacea |
| 4 | I worry that my rosacea might get worse |
| 5 | I worry about the side effects associated with rosacea drugs |
| 6 | My rosacea is irritated |
| 7 | My rosacea embarrasses me |
| 8 | I am frustrated with my rosacea |
| 9 | My rosacea makes my skin sensitive |
| 10 | I am annoyed by my rosacea |
| 11 | I am bothered by the look of my skin (redness, spots) |
| 12 | My rosacea makes me feel self-conscious |
| 13 | I try to cover my rosacea (with makeup) |
| 14 | I am bothered by the persistence/recurrence of my rosacea |
| 15 | I avoid certain foods and drinks due to my rosacea |
| 16 | My skin looks bumpy (uneven, not smooth, irregular) |
| 17 | My skin gets red |
| 18 | My skin gets easily irritated (cosmetics, aftershave lotions, makeup removers) |
| 19 | My eyes bother me (they feel dry or gritty) |
| 20 | I think about my rosacea |
| 21 | I avoid certain environments (heat, humidity, cold) due to my rosacea |
According to the IGA scale, IGA grade 2 was the most common one found in 44.4% of the cases (Table 2).
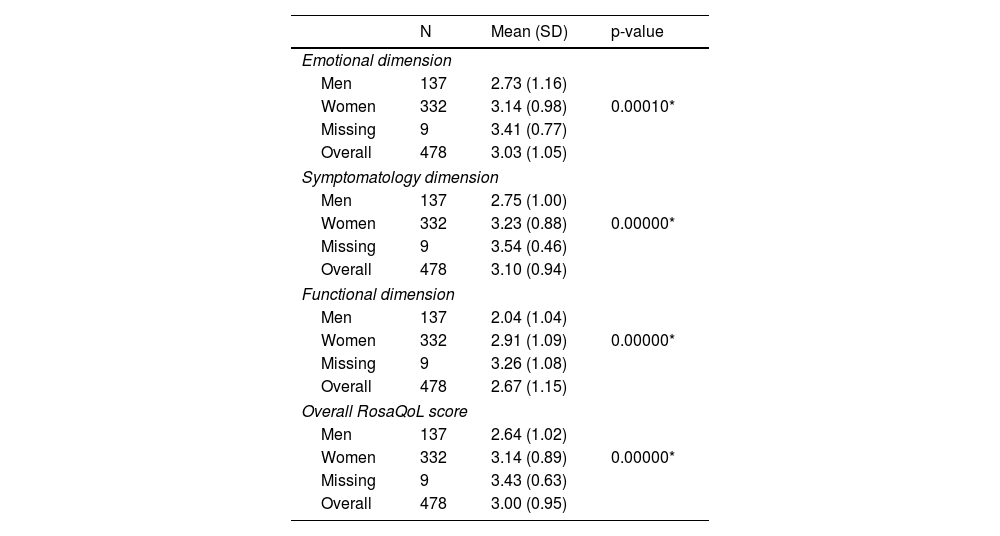
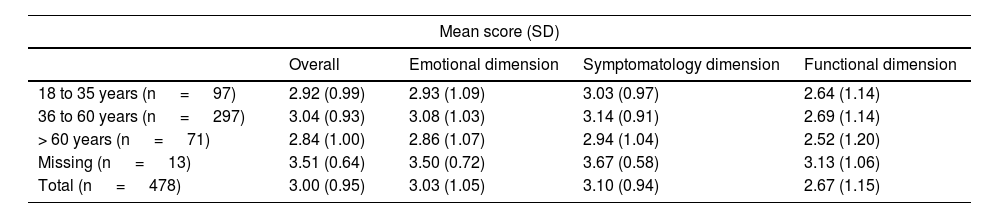
Statistically significant differences were reported between men and women in the overall score of the RosaQoL questionnaire, as well as in each of the 3 dimensions of the scale (symptoms, functionality, and emotional state; p <0.001) (Table 3), although these differences were non statistically significant when analyzed by age (Table 4).
Differences in the RosaQoLquestionnaire scores at initial visit by gender.
| N | Mean (SD) | p-value | |
|---|---|---|---|
| Emotional dimension | |||
| Men | 137 | 2.73 (1.16) | |
| Women | 332 | 3.14 (0.98) | 0.00010* |
| Missing | 9 | 3.41 (0.77) | |
| Overall | 478 | 3.03 (1.05) | |
| Symptomatology dimension | |||
| Men | 137 | 2.75 (1.00) | |
| Women | 332 | 3.23 (0.88) | 0.00000* |
| Missing | 9 | 3.54 (0.46) | |
| Overall | 478 | 3.10 (0.94) | |
| Functional dimension | |||
| Men | 137 | 2.04 (1.04) | |
| Women | 332 | 2.91 (1.09) | 0.00000* |
| Missing | 9 | 3.26 (1.08) | |
| Overall | 478 | 2.67 (1.15) | |
| Overall RosaQoL score | |||
| Men | 137 | 2.64 (1.02) | |
| Women | 332 | 3.14 (0.89) | 0.00000* |
| Missing | 9 | 3.43 (0.63) | |
| Overall | 478 | 3.00 (0.95) | |
Results of the RosaQoL Questionnaire scores by age groups (cases only).
| Mean score (SD) | ||||
|---|---|---|---|---|
| Overall | Emotional dimension | Symptomatology dimension | Functional dimension | |
| 18 to 35 years (n = 97) | 2.92 (0.99) | 2.93 (1.09) | 3.03 (0.97) | 2.64 (1.14) |
| 36 to 60 years (n = 297) | 3.04 (0.93) | 3.08 (1.03) | 3.14 (0.91) | 2.69 (1.14) |
| > 60 years (n = 71) | 2.84 (1.00) | 2.86 (1.07) | 2.94 (1.04) | 2.52 (1.20) |
| Missing (n = 13) | 3.51 (0.64) | 3.50 (0.72) | 3.67 (0.58) | 3.13 (1.06) |
| Total (n = 478) | 3.00 (0.95) | 3.03 (1.05) | 3.10 (0.94) | 2.67 (1.15) |
p-value not significant for all age groups.
No significant differences were found in the RosaQoL score between the control group (healthy patients [n=50], mean overall score, 43.5 [SD, 13.7]) and the patient group ([n=468], mean overall score, 47.3 [SD, 13.3]; p=0.052).
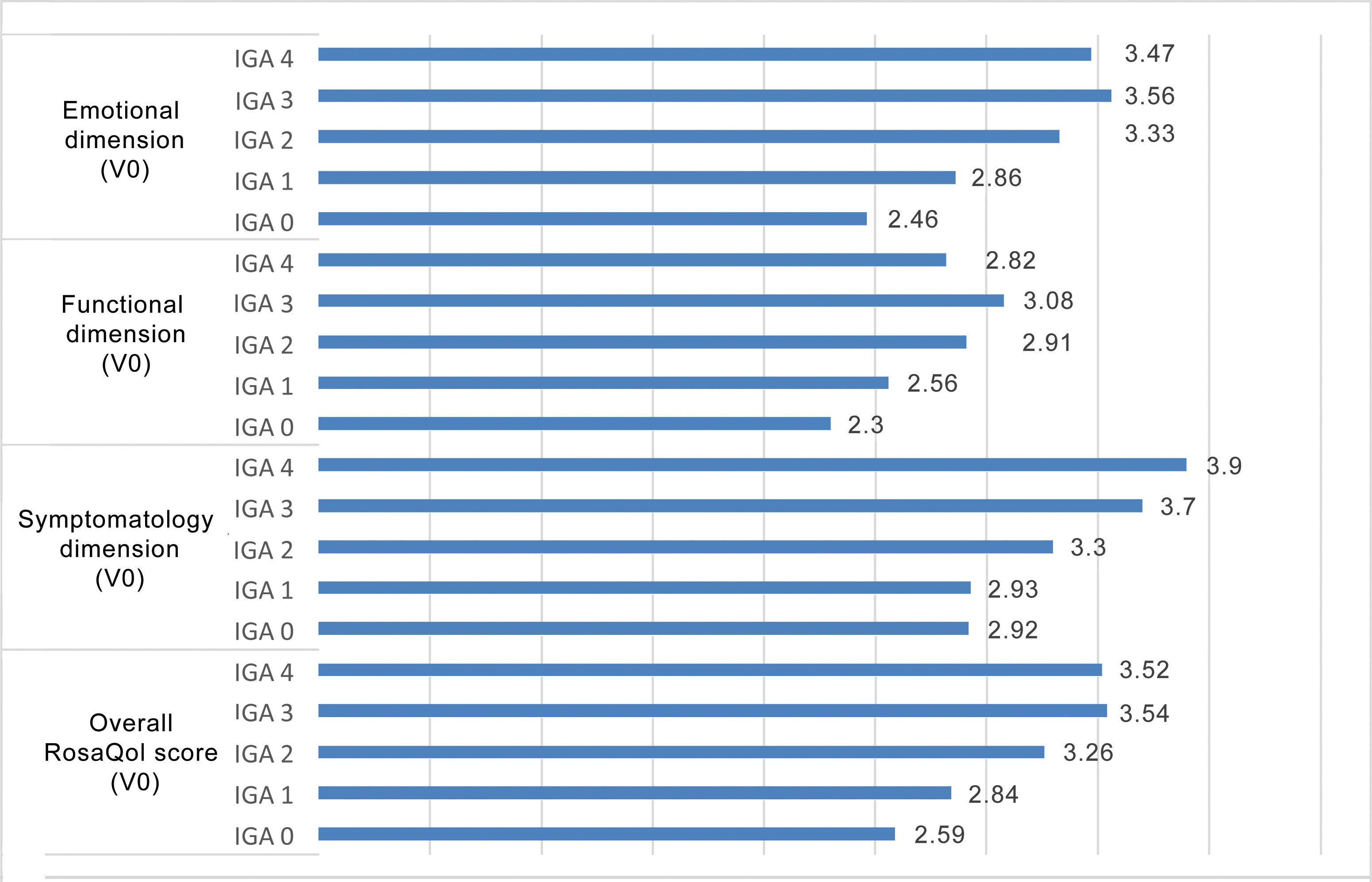
Similarly, the score obtained in RosaQoL was higher in more severe grades of rosacea, with a greater impact being reported in the symptomatology dimension (Figure 1).
The internal consistency method, based on Cronbach's alpha, estimates the reliability of a measurement instrument through a set of items expected to measure the same construct or theoretical dimension. Cronbach's alpha value evaluated across all questionnaires was 0.96, which is consistent with the general criterion recommended by George and Mallery21, where values > 0.9 are indicative of high internal consistency.
When each item was individually removed from the scale, no improvement in Cronbach's alpha was obtained. Therefore, it is established that no item is prone to elimination. The questionnaire statistical power to discriminate cases using the ROC curve yielded an area under the curve (AUC) of 0.96 (95%CI, 0.92-0.99). Considering that values > 0.9 indicate high discriminative power, we can say that the questionnaire has a high capacity to detect cases.
The evaluation of sensitivity and specificity using Youden's criterion yielded a cutoff in the RosaQoL score > 1.476, thusproviding the questionnaire with high sensitivity, with a value of 0.99, and a specificity to detect cases of 0.85. For this cutoff, 425 true positives, 43 true negatives, 7 false positives, and 3 false negatives were reported.
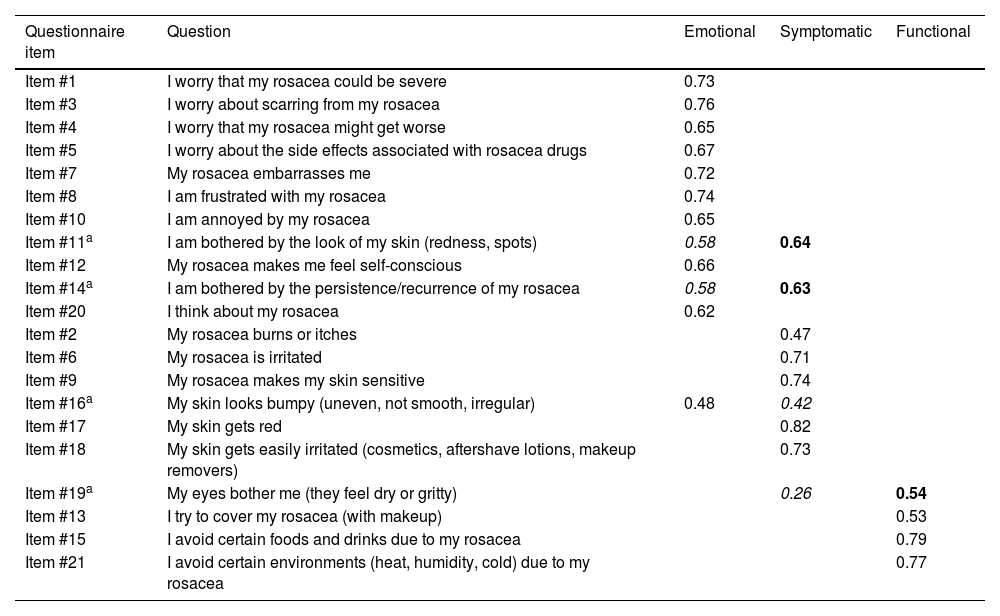
The Varimax rotated component matrix for 3 factors contributes to the validation of the scale construct, showing that items tend to group into the functional, emotional, and symptomatic factors proposed in the original scale26 (Table 5).
Rotated component matrix of the varimax factor model for the RosaQoL questionnaire (cases only).
| Questionnaire item | Question | Emotional | Symptomatic | Functional |
|---|---|---|---|---|
| Item #1 | I worry that my rosacea could be severe | 0.73 | ||
| Item #3 | I worry about scarring from my rosacea | 0.76 | ||
| Item #4 | I worry that my rosacea might get worse | 0.65 | ||
| Item #5 | I worry about the side effects associated with rosacea drugs | 0.67 | ||
| Item #7 | My rosacea embarrasses me | 0.72 | ||
| Item #8 | I am frustrated with my rosacea | 0.74 | ||
| Item #10 | I am annoyed by my rosacea | 0.65 | ||
| Item #11a | I am bothered by the look of my skin (redness, spots) | 0.58 | 0.64 | |
| Item #12 | My rosacea makes me feel self-conscious | 0.66 | ||
| Item #14a | I am bothered by the persistence/recurrence of my rosacea | 0.58 | 0.63 | |
| Item #20 | I think about my rosacea | 0.62 | ||
| Item #2 | My rosacea burns or itches | 0.47 | ||
| Item #6 | My rosacea is irritated | 0.71 | ||
| Item #9 | My rosacea makes my skin sensitive | 0.74 | ||
| Item #16a | My skin looks bumpy (uneven, not smooth, irregular) | 0.48 | 0.42 | |
| Item #17 | My skin gets red | 0.82 | ||
| Item #18 | My skin gets easily irritated (cosmetics, aftershave lotions, makeup removers) | 0.73 | ||
| Item #19a | My eyes bother me (they feel dry or gritty) | 0.26 | 0.54 | |
| Item #13 | I try to cover my rosacea (with makeup) | 0.53 | ||
| Item #15 | I avoid certain foods and drinks due to my rosacea | 0.79 | ||
| Item #21 | I avoid certain environments (heat, humidity, cold) due to my rosacea | 0.77 |
Figures in bold show the results of the factor analysis in the dimension in which it is categorized within the Spanish scale.
Figures in italics show the results of the factor analysis in the dimension it occupied in the original scale.
All the items of the questionnaire are categorized as in the original questionnaire, except for slight discrepancies in 4 of them. Item #11 “I am bothered by the look of my skin (redness, spots)” and item #14 “I am bothered by the persistence/recurrence of my rosacea,” which in the original scale are grouped in the emotional dimension and seem to have a slightly more specific weight in the symptomatic dimension in the Spanish version. Although item #16 “My skin looks bumpy (uneven, not smooth, irregular)” should be represented in the symptomatic dimension, it is somehow better represented in the emotional one. Finally, although item #19 “My eyes bother me (I can feel how dry or gritty they are),” should be better represented in the symptomatic dimension, it was better represented in the functional one. Since the specific weight difference of these 4 items was minimal, we decided to maintain the original dimension classification for comparative evaluation purposes with other versions of the scale.
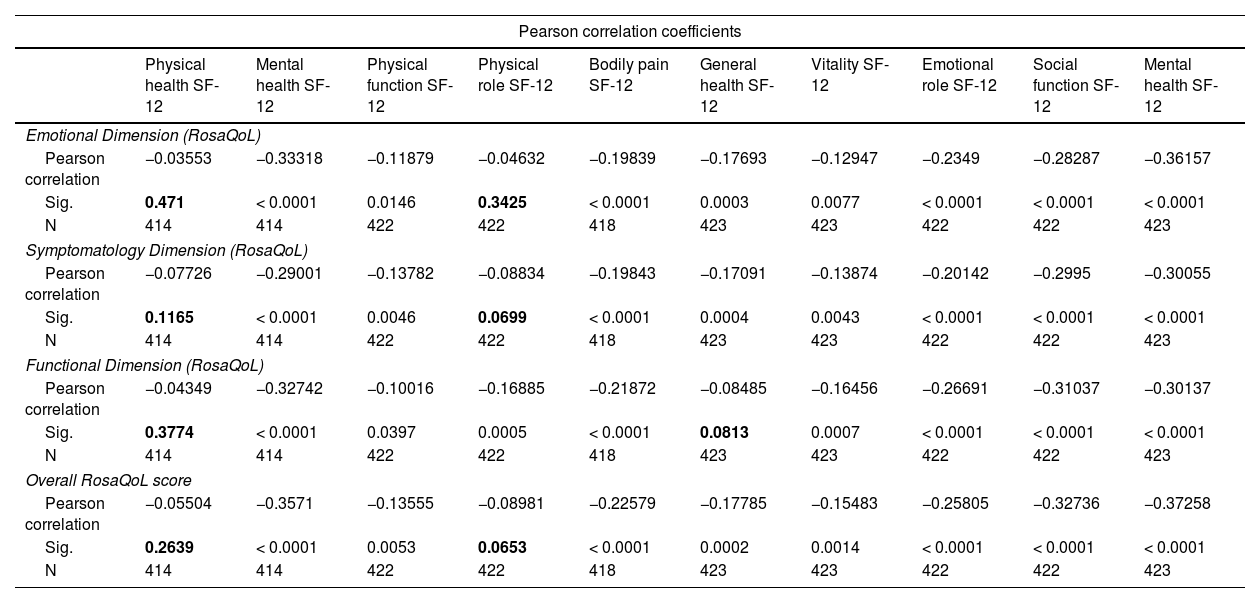
When analyzing the correlation between the RosaQoL scale and the more generalist SF-12 scale, most studied correlations were statistically significant and entirely negative. A higher score on the RosaQoL scale in any dimension (whether functional, emotional, or symptomatic) is associated with a worse quality of life in all dimensions of the SF-12 scale (Table 6).
Correlation between the RosaQoL and SF-12 scales.
| Pearson correlation coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical health SF-12 | Mental health SF-12 | Physical function SF-12 | Physical role SF-12 | Bodily pain SF-12 | General health SF-12 | Vitality SF-12 | Emotional role SF-12 | Social function SF-12 | Mental health SF-12 | |
| Emotional Dimension (RosaQoL) | ||||||||||
| Pearson correlation | −0.03553 | −0.33318 | −0.11879 | −0.04632 | −0.19839 | −0.17693 | −0.12947 | −0.2349 | −0.28287 | −0.36157 |
| Sig. | 0.471 | < 0.0001 | 0.0146 | 0.3425 | < 0.0001 | 0.0003 | 0.0077 | < 0.0001 | < 0.0001 | < 0.0001 |
| N | 414 | 414 | 422 | 422 | 418 | 423 | 423 | 422 | 422 | 423 |
| Symptomatology Dimension (RosaQoL) | ||||||||||
| Pearson correlation | −0.07726 | −0.29001 | −0.13782 | −0.08834 | −0.19843 | −0.17091 | −0.13874 | −0.20142 | −0.2995 | −0.30055 |
| Sig. | 0.1165 | < 0.0001 | 0.0046 | 0.0699 | < 0.0001 | 0.0004 | 0.0043 | < 0.0001 | < 0.0001 | < 0.0001 |
| N | 414 | 414 | 422 | 422 | 418 | 423 | 423 | 422 | 422 | 423 |
| Functional Dimension (RosaQoL) | ||||||||||
| Pearson correlation | −0.04349 | −0.32742 | −0.10016 | −0.16885 | −0.21872 | −0.08485 | −0.16456 | −0.26691 | −0.31037 | −0.30137 |
| Sig. | 0.3774 | < 0.0001 | 0.0397 | 0.0005 | < 0.0001 | 0.0813 | 0.0007 | < 0.0001 | < 0.0001 | < 0.0001 |
| N | 414 | 414 | 422 | 422 | 418 | 423 | 423 | 422 | 422 | 423 |
| Overall RosaQoL score | ||||||||||
| Pearson correlation | −0.05504 | −0.3571 | −0.13555 | −0.08981 | −0.22579 | −0.17785 | −0.15483 | −0.25805 | −0.32736 | −0.37258 |
| Sig. | 0.2639 | < 0.0001 | 0.0053 | 0.0653 | < 0.0001 | 0.0002 | 0.0014 | < 0.0001 | < 0.0001 | < 0.0001 |
| N | 414 | 414 | 422 | 422 | 418 | 423 | 423 | 422 | 422 | 423 |
Figures in bold show statistically non-significant correlations.
The temporal reliability of the questionnaire was confirmed through the test-retest method, as no discrepancies were detected over time between patients’ responses.
To evaluate sensitivity to change, a follow-up visit was conducted 3 months after the start of the study, when rosacea patients were asked to complete both the RosaQoL and SF-12 questionnaires again. In most calculated correlations, a statistically significant negative correlation was reported. A greater increase in the RosaQoL scale and all its dimensions (functional, emotional, and symptomatic) was associated with a worse progression of quality of life in all dimensions of the SF-12 scale. This same correlation was also seen when analyzed by age group and disease severity.
DiscussionTo consider a scale valid as an instrument for measuring HRQoL, a simple translation is not sufficient; rather, a series of structured and guided steps are necessary.13 To make sure that the reliability and validity requirements of such tools are met, the measurement properties of the translated version in a population need to be evaluated with similar characteristics and then submitted to a validation process.25
The study of measurement properties demonstrates that the Spanish-translated and culturally adapted version of the RosaQoL questionnaire allows us, like the original version, to obtain statistically significant differences between the healthy population and the population with rosacea. The level of impact on the RosaQoL scale in all dimensions was directly proportional to the severity of rosacea, with the highest degree of impact being reported in the symptomatology dimension.
Nicholson et al.11 highlighted the validity of RosaQoL in discriminating rosacea patients vs the SKINDEX-29. In its original version, the specific RosaQoL scale showed a higher response level at 4-6 months vs the SKINDEX-29 for the total score in patients reporting improvement in their rosacea, showing greater specificity.
The reliability study of the RosaQoL questionnaire demonstrates that the tool has a high degree of internal consistency, obtaining a Cronbach's alpha value of 0.96, and that its discriminative power to detect cases is extremely high, obtaining a ROC AUC score of 0.96.
Reproducibility is one of the important points we should consider when evaluating a tool for measuring HRQoL. To assess reproducibility, it is checked whether similar scores can be obtained when applied in 2 different points in time to the same population by the same evaluators using the exact same method. Since it is important to avoid the “learning effect,” the time between the initial test and the retest should not be too long.27 In our study, the score obtained with the RosaQoL questionnaire does not vary significantly between the test and retest results of the same patient (interval of 1 to 2 days). Our test-retest results show a very high correlation between both tests in the 3 dimensions of the questionnaire, indicating a high degree of reliability.
The construct validity study through factor analysis showed that the items of the RosaQoL scale tend to group based on the functional, emotional, and symptomatic dimensions, as in the original version of the scale, and that the items that make up each one of them tend to group consistently in each dimension.
Our study also analyzed the convergence (“correlation”) between the RosaQoL scale and the more generalist SF-12 scale and found that it was positive, evidencing that the scales are conceptually congruent or similar:25 higher increases in the RosaQoL scale and all its sub-dimensions (functional, emotional, and symptomatic) were associated with worse quality of life in all dimensions of the SF-12 scale.
ConclusionsThe RosaQoL scale, adapted to Spanish, has proven to be a valid, sensitive, and reliable tool for measuring quality of life, in all its dimensions (symptoms, functionality, and emotional state) among the Spanish population with rosacea. Results indicate that the greater the severity of rosacea, the greater the impact on quality of life, with the dimensions of symptomatology and emotional state being the most affected ones, especially in women.
Conflicts of interestThis project has received funding and support from Laboratorios Galderma S.A.U. Dr. Salleras and Dr. Pozo declared no conflicts of interest whatsoever. Dr. Ribera received grants and fees related to research, consulting, and training from the following companies: AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Galderma, Gebro Pharma, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Pierre-Fabre, Sandoz, SKB, and UCB.
We wish to thank all group participants for their collaboration while working on this manuscript, Laboratorios Galderma S.A.U. for funding the project, and CRO Crossdata for their advice while working on the project and writing this article.