Immune checkpoint inhibitors (ICIs) have significantly advanced the treatment of cancer. They are not, however, free of adverse effects. These effects are called immune-related adverse events (irAEs) and often involve the skin. Most of the information on cutaneous irAEs comes from clinical practice. We therefore conducted a thorough review of the characteristics of cutaneous irAEs, recommendations for treatment, and their association with prognosis. The most common events are exanthema, pruritus, vitiligo, and hair loss, although ICIs can cause a wide range of cutaneous dermatoses. The reported association observed between certain reactions and a favorable response to cancer treatment should be interpreted with caution. Dermatologists should be involved in the multidisciplinary care of patients being treated with ICIs as they have an essential role in the diagnosis and treatment of cutaneous irAEs.
Los fármacos inhibidores de los puntos de control inmunitario han supuesto un importante avance en el tratamiento oncológico. Sin embargo, su uso no está exento de reacciones no deseadas, denominadas efectos adversos inmunorrelacionados, siendo los cutáneos particularmente frecuentes. El conocimiento que tenemos sobre los efectos adversos inmunorrelacionados cutáneos procede fundamentalmente de la práctica clínica. Por lo tanto, en este trabajo se revisan en detalle sus características, así como las recomendaciones sobre su tratamiento y sus implicaciones pronósticas. Los más frecuentes son el exantema, el prurito, el vitíligo y la alopecia; sin embargo, estos fármacos pueden producir una amplia variedad de dermatosis. La asociación observada entre ciertos tipos de reacciones cutáneas con una respuesta oncológica favorable al tratamiento debe interpretarse con cautela. El dermatólogo ha de participar en el cuidado multidisciplinar de estos pacientes, pues desempeña un papel fundamental en el diagnóstico y el tratamiento de estas reacciones cutáneas adversas.
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of cancer. Their demonstrated efficacy and association with longer survival times have been demonstrated in a broad spectrum of advanced tumors.1–4
ICIs stimulate the immune system, activating tumor-destroying T cells, but as a consequence of this stimulus, diverse autoimmune or autoinflammatory events can be triggered.4–7 Known as immune-related adverse events (irAEs), they can appear in any organ or tissue, but among the most frequently described are dermatologic toxicities, found in approximately a third of patients on these drugs.4,7
Information from clinical trials on the incidence and profiles of dermatologic irAEs is difficult to evaluate because cutaneous toxicity is usually recorded in generical terms in clinical trials.8,9 Our current understanding of these reactions therefore comes mainly from clinical practice as reflected in retrospective studies, case series, and individual case reports, with all the limitations those sources imply.4,5,9
Our aim was to review and synthesize the literature on irAEs in patients being treated with ICIs, describe the reactions, the drug regimens used, and the prognostic implications.
MethodsWe used the terms immune checkpoint inhibitors and skin toxicity to search PubMed and the Web of Science for the period from January 2015 to May 2021. We also searched the Cochrane Library without specifying time limits. Two authors (G.J.C. and M.B.M.) independently reviewed titles and abstracts to select articles with information on the frequency and characteristics of cutaneous irAEs in patients with ICI-treated cancer, on the treatments that are used, or on prognosis. Other relevant articles were identified by searching the reference lists of the retrieved articles.
Relevant articles could report any study design and be published in English or Spanish provided they included data on the number or percentages of patients on ICIs, measured associations or survival rates, or at least described in detail the observed skin eruptions (including type of rash, type of ICIs, and latency from the start of treatment). Any type of ICI in monotherapy or in combination was of interest for the review. Two authors (G.J.C. and M.B.M.) independently read the full texts of the selected articles. Articles without abstracts were also read in full. We included the most up-to-date articles, excluding older ones whose results were included in more recent publications or that had redundant information.
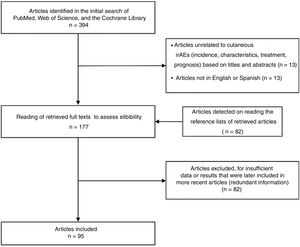
ResultsThe literature search suggested a total of 394 articles. Ninety-five with up-to-date information were selected for the final review (Fig. 1). Among those chosen were 2 systematic reviews, 4 meta-analyses, and 1 clinical trial. The remaining references were retrospective studies, narrative reviews, and case reports or case series.
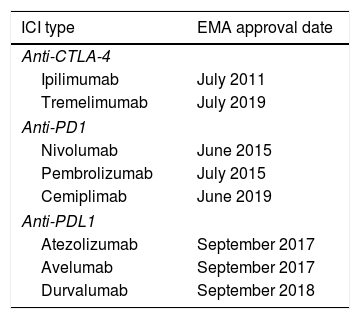
Pathogenesis and FrequencyTwo groups of ICIs are currently available: cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors and programmed cell death protein 1 (PD1) or ligand 1 (PDL1) inhibitors (Table 1). The CTLA-4 receptor controls the immune response in stages prior to the PD1/PDL1 pathway, which regulates later stages of response, mainly involving peripheral tissues.1 The pathogenesis of irAEs is not yet fully understood. However, although it is known that the activation of CD4+ and CD8+ T cells produced by blocking PD1 and PDL1 is beneficial from the perspective of cancer treatment due to the effect on tumor cells, their inhibition also plays a fundamental role in the development of irAEs in general and cell toxicity in particular.1
ICI Types and Dates of Approval by the EMA.
| ICI type | EMA approval date |
|---|---|
| Anti-CTLA-4 | |
| Ipilimumab | July 2011 |
| Tremelimumab | July 2019 |
| Anti-PD1 | |
| Nivolumab | June 2015 |
| Pembrolizumab | July 2015 |
| Cemiplimab | June 2019 |
| Anti-PDL1 | |
| Atezolizumab | September 2017 |
| Avelumab | September 2017 |
| Durvalumab | September 2018 |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; EMA, European Medicines Agency; ICI, immune checkpoint inhibitor; PD1, programmed cell death protein 1; PDL1, ligand of PD1.
Even though all ICIs have similar safety profiles, there are differences in irAE type, frequency, latency, and seriousness associated with each ICI, given that molecular targets and pharmacokinetic characteristics differ from one to another.5,6
Ipilimumab induces dose-dependent skin irAEs more often than PD1/PDL1 blockers (in 50% vs 10%–30%) of cases.2,8–12 In addition, ipilimumab-associated events occur earlier after start of treatment and are more severe.1–3 Ipilimumab in combination with PD1/PDL1 blockers has been linked to the highest incidences of cutaneous toxicity of any degree of severity, especially if pembrolizumab is part of the regimen.6,9 PD1 inhibitors (mainly pembrolizumab) confer higher risk of dermatologic irAEs than PDL1 inhibitors do, whereas avelumab is associated with lower risk.5
It is important to point out that various irAEs can coincide in the same patient and that multisystem irAEs develop in up to 9% of those treated. Common associations are dermatitis–pneumonitis and dermatitis–thyroiditis. Skin toxicities often appear first.10
Associations between type of irAE and tumor type have recently been suggested, given that more cases of skin irAEs have been reported in patients with melanoma than other tumors.4 However, we think caution is called for when interpreting this association, as it could be a product of reporting bias, arising from the fact that dermatologists are usually involved in the treatment and follow-up of melanoma.
Types of Cutaneous Toxicity and CharacteristicsThe irAEs most often reported in clinical trials are exanthema (rash or dermatitis), pruritus, vitiligo, and hair loss.4,5,8,9 However, more diverse ICI-induced dermatologic toxicities are found in clinical practice. We base this review on the classification most commonly used at this time.13,14 It consists of the following 4 large groups: inflammatory conditions, immunobullous conditions, alterations of keratinocytes, and alterations of epidermal melanocytes. Some authors, however, consider these categories to be imprecise and have modified them. We will group melanocytic changes in a larger category of pigmentary alterations and also add 2 sections: hair and nail involvement and other rare dermatoses.11
The severity of toxicities is usually evaluated with the Common Terminology Criteria for Adverse Events (CTCAE), which specifies 4 grades according to the affected body surface area (BSA) (Table 2).13,15,16 Some authors, however, recommend also taking the nature of a dermatosis into consideration in order to more precisely characterize the severity of the clinical picture.13,15,17,18
Severity of Skin irAEs Graded According to the CTCAE and Exemplified by Maculopapular Rashes.
| Grade | Characteristics |
|---|---|
| 1 | Covering<10% BSA, with or without symptoms (pruritus, burning, tightness) |
| 2 | Covering 10%–30% BSA, with or without symptoms; ADL interference |
| 3 | Covering>30% BSA, with or without symptoms; ADL interference |
| 4 | SJS, TEC, bullous dermatitis covering>30% BSA requiring hospitalization and ICU admission |
Source: Haanen et al.13
Abbreviations: ADL, activities of daily living; BSA, body surface area; CTCAE, Common Terminology Criteria for Adverse Events; ICU, intensive care unit; TEC, toxic epidermal necrolysis; SJS, Stevens–Johnson syndrome.
Along with pruritus, maculopapular rashes are the most common irAEs. They develop in approximately 25% of ipilimumab-treated patients, 15% of those on anti-PD1 antibody treatments, and 10% of those on anti-PDL1 drugs. Up to 45% of patients on combined anti-CTLA-4/PD1 therapy can be affected, however.9,13,18 The rashes tend to be mild: fewer than 3% of cases are rated grade 3 or higher in severity.9
The eruption typically develops early, in the first 2 to 6 weeks of treatment, although it may also appear later. The fairly nonspecific clinical signs are confluent, pruritic maculopapular lesions on the trunk and sometimes on the extremities. Peripheral blood eosinophilia may be detected.18,19
The most common histologic pattern is that of a spongiotic dermatitis with a superficial perivascular lymphocytic infiltrate with eosinophils, although a lichenoid pattern has also been reported on occasion.18,20,21
Because this irAE is mild, it can usually be managed with symptomatic therapy (oral antihistamines and topical corticosteroids), even when 30% of the BSA is covered; if the rash is refractory to topical applications, systemic corticosteroids are needed.13,15,16,18,22
A maculopapular rash may precede other skin conditions. Follow-up is therefore necessary, and clinically atypical, severe, persistent, or recurrent lesions should be biopsied.11,17,18,22–25
PruritusPruritus is one of the most prevalent irAEs, presenting in up to 32% of patients on ICIs.26 Itching may be associated with other dermatoses, be the first sign of more severe irAEs such as bullous pemphigoid, or be an isolated event indicating increased activation of the skin's immune system.
If xerosis is present, the pruritus must be treated. When itching is mild or intermittent (grades 1–2), topical corticosteroids, oral antihistamines, and emollient creams are recommended. In cases in which symptoms are difficult to control and greatly impair the patient's quality of life (grade 3), treatments that have been used include γ-aminobutyric acid A receptor antagonists, aprepitant, phototherapy, naloxone, naltrexone, omalizumab, and dupilumab. The reported results vary. Treatment with the culprit drug must be suspended on rare occasions.13,22,26–29
Lichenoid EruptionsLichenoid reactions have been reported mainly in association with anti-PD1/PDL1 drugs, and according to some authors these rashes have the histopathologic pattern most often seen in ICI-associated irAEs.19,21,23,30,31
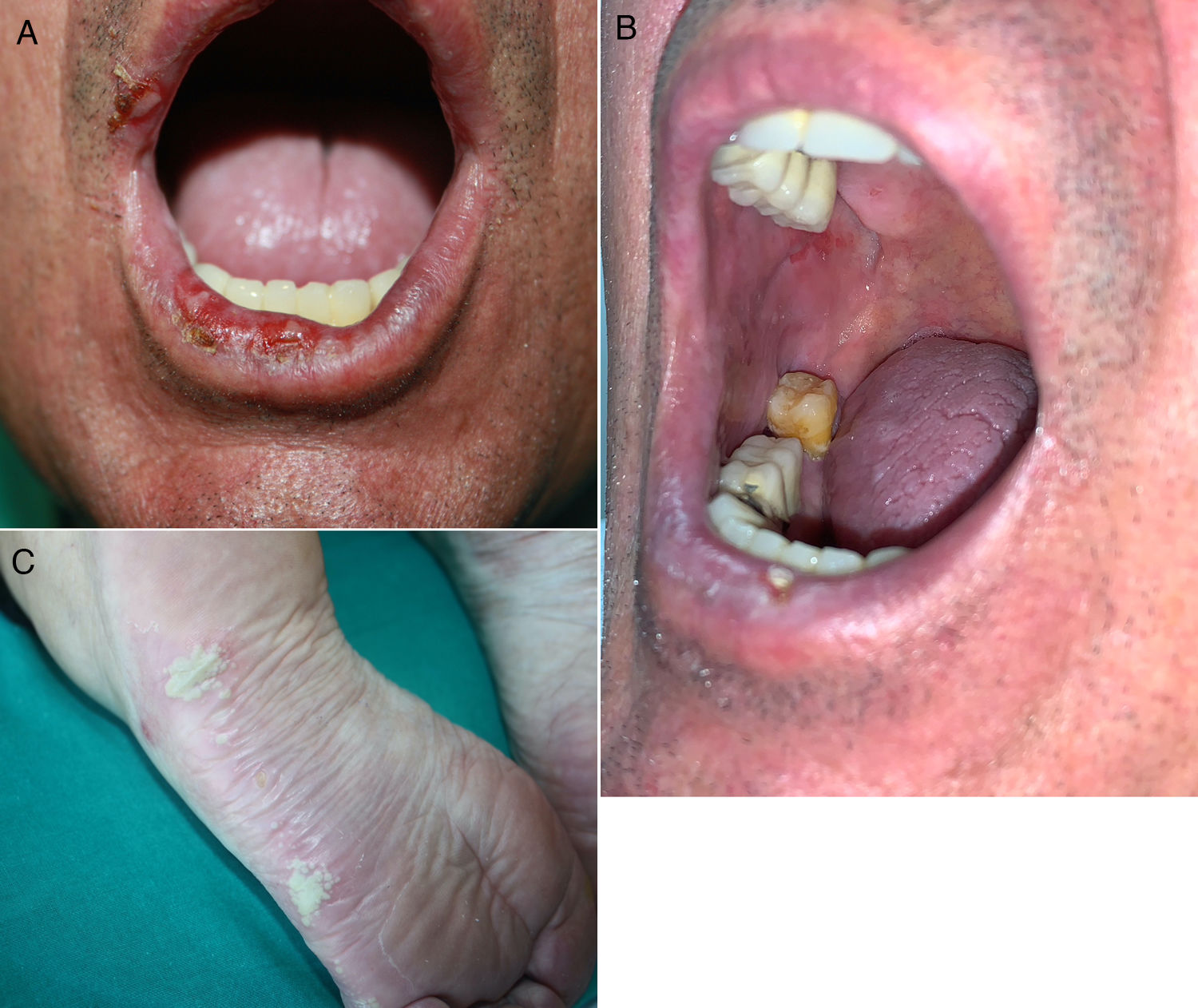
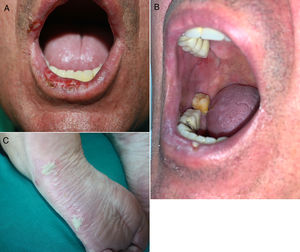
Lichenoid eruptions develop later than maculopapular rashes, appearing at 3 months from the start of treatment on average (range, 1 day to 14 months).23,31,32 Clinical signs vary from forms resembling lichen planus to more atypical presentations with hypertrophic or erosive lesions. They may also resemble lichen planus pemphigoides or lichen sclerosus. Nail alterations are sometimes observed.21,23,31,33,34 Pruritus is a common symptom and may be difficult to treat.35 Mucosal involvement is not unusual and may be the only presentation, in the form of whitish striae or erosive or atrophic lesions (Fig. 2, A and B). Characteristics may sometimes overlap with those of eczematous dermatitis or resemble a maculopapular rash. In such cases a definitive histopathologic diagnosis is required,18,21,23,31,33,34 meaning that the incidence of lichenoid dermatitis may be underestimated.17,20,35
Inflammatory dermatoses. A and B, Erosive oral lesions in a 60-year-old man with stage IV adenocarcinoma of the bronchi and lungs in treatment with durvalumab. Histology demonstrated lichenoid dermatitis. C, Plantar pustulosis in a 68-year-old woman with stage IV adenocarcinoma of the bronchi and lungs in treatment with pembrolizumab.
Biopsy can demonstrate the classic changes of lichen planus: a band-like lymphocytic infiltrate, hypergranulosis, and irregular acanthosis. However, spongiosis, parakeratosis, eosinophils, or a slight degree of interface dermatitis may be evident, consistent with a diagnosis of lichenoid dermatitis.20,21,23,33
Corticosteroids are the first line of treatment. Even rashes covering a large area respond well. If the eruption is refractory, systemic corticosteroids, phototherapy, oral acitretin, or even methotrexate or apremilast may be prescribed. Treatment with the culprit drug generally need not be suspended; in some cases therapy has been restarted without recurrence of the reaction.17,23,33,35
Lichenoid eruptions have been associated with a good response to oncologic therapy.32
Eczematous EruptionsEczemas occur mainly in patients on anti-PD1/PDL1 inhibitors.14,19,30
They typically present later than maculopapular rashes, usually after 3 months of treatment.19,32 However, they may appear up to 2 years after therapy.30 Lesions may be generalized or local and are usually accompanied by pruritus.19,30 On biopsy, spongiosis can be seen in the epidermis and a perivascular inflammatory infiltrate in the dermis.20
Depending on severity, topical or systemic corticosteroids, topical tacrolimus, oral antihistamines, or UV-B phototherapy may be prescribed.30
PsoriasisReports of both de novo and exacerbated psoriasis associated with anti-PD1 inhibitors, and less often with anti-PDL1 blockers and ipilimumab, have been published.36 Mean latency after start of therapy ranges from 1 to 8 months. Exacerbations present earlier than de novo cases.36–38
The most common form of presentation is plaque psoriasis, followed by palmoplantar psoriasis. Pustular forms (Fig. 2C) have been documented as have guttate, inverse, erythrodermic, and nail psoriasis, as well as sebopsoriasis, combinations of subtypes, and associations with psoriatic arthritis.37–40
Histologic findings tend to be those typical of eczema, although a degree of spongiosis has also been reported, especially in inverse psoriasis lesions.19,20
The pathogenic mechanisms are unclear, but it appears that PD1 inhibition activates Th1 and Th17 pathways with consequent overexpression of interferon-γ, IL-2, tumor necrosis factor, IL-6, and IL-17.14,20,36
Symptoms are usually mild (BSA≤10%) in most patients and respond well to high-potency topical corticosteroids combined with calcipotriol.36,38 Adding phototherapy (narrowband UV-B) or acitretin is recommended if there is no response. Refractory cases can be treated with methotrexate, apremilast, or biologics (preferably anti-tumor necrosis factor agents) as a last resort; results vary.17,38,39 Systemic corticosteroids have been used, but they are best reserved for achieving a rapid response or after other measures have failed.38,39 The culprit drug must be suspended temporarily or definitively in fewer than half the cases.36,38
Sarcoidosis-Like Granulomatous EruptionsGranulomatous dermatitis resembling sarcoidosis appears in a variable percentage of patients on ICIs—ranging from 0.65% to 22% in different series.41–43 These reactions have been reported with the use of anti-PD1/PDL1 antibodies and ipilimumab.18,41,43
Latency ranges from 1.5 to 7 months after start of therapy, although reactions can develop several months after treatment stopped. The organs most commonly affected are the mediastinal and hilar lymph nodes, the lungs, and the skin. Skin signs consist of erythematous papules or nodules coalescing into plaques; the lesions are pruritic, sometimes painful, and located on the face or extremities.41–43 Histology demonstrates nonnecrotizing granulomas.20,41
A proliferation of Th1 and Th17 cells induced by anti-CTLA-4 antibodies has been reported. The adverse reaction could be paradoxical, however, given that patients with sarcoidosis have higher expression of PD1 in T cells. Thus, blocking the PD1 receptor could be considered a therapeutic strategy in this disease.20,41,43
Skin lesions are treated with high-potency topical corticosteroids. If the response is unsatisfactory, systemic corticosteroids are prescribed. Oral hydroxychloroquine has occasionally been used.41 Lymph node and lung involvement must be ruled out. When skin lesions are persistent or extensive, radiologic signs progress, lung function deteriorates, or other organs are affected, systemic corticosteroids should be started and the culprit ICI suspended. Once the corticosteroid dose has been reduced to 10mg/kg/d or less and the patient is asymptomatic, restarting ICI therapy can be considered.41–43
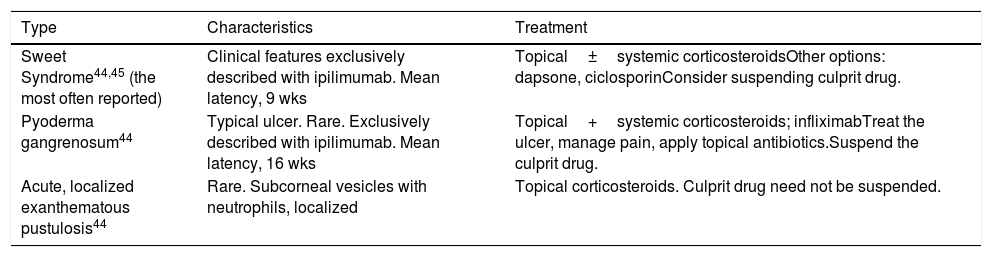
Neutrophilic DermatosesSeveral types of neutrophilic dermatoses, mainly Sweet syndrome, have been linked to ICIs. The characteristics of these irAEs are given in Table 3.44,45
ICI-Associated Neutrophilic Dermatoses.
| Type | Characteristics | Treatment |
|---|---|---|
| Sweet Syndrome44,45 (the most often reported) | Clinical features exclusively described with ipilimumab. Mean latency, 9 wks | Topical±systemic corticosteroidsOther options: dapsone, ciclosporinConsider suspending culprit drug. |
| Pyoderma gangrenosum44 | Typical ulcer. Rare. Exclusively described with ipilimumab. Mean latency, 16 wks | Topical+systemic corticosteroids; infliximabTreat the ulcer, manage pain, apply topical antibiotics.Suspend the culprit drug. |
| Acute, localized exanthematous pustulosis44 | Rare. Subcorneal vesicles with neutrophils, localized | Topical corticosteroids. Culprit drug need not be suspended. |
Abbreviation: ICI, immune checkpoint inhibitor.
The frequency of serious skin reactions to ICIs is low, affecting fewer than 3% of patients on these drugs,5,9,14,24 but cases of Stevens–Johnson syndrome, toxic epidermal necrolysis (TEC), DRESS (drug reaction with eosinophilia and systemic symptoms), and acute generalized exanthematous pustulosis have been reported.14,24,44,46–48
ICI-induced cases of TEC can have atypical, late presentations that develop up to 12 weeks after therapy started. These irAEs begin as maculopapular eruptions and persist for weeks until blistering and epidermal detachment appear.18,24,48,49
Biopsy for direct immunofluorescence is indicated in such cases to rule out an immunobullous reaction.48 The culprit agent must be suspended, the patient hospitalized, and life support measures initiated. Systemic corticosteroids are recommended to treat ICI-induced TEC, unlike TEC induced by other drugs. Treatment continues until symptoms improve to grade 1, at which point the dose is gradually reduced.13,15,16,24,48 Intravenous infliximab, ciclosporin, and immunoglobulins have been used.17,24,49 Mortality can be as high as 50%–60%.48,49
Eruptions Resembling Connective Tissue DiseasesConnective tissue diseases associated with ICIs are emerging toxicities.50–55 De novo diseases account for 0.025% of the observations in patients on ICI therapy, and the incidence is similar in men and women. They develop mainly in the context of treatment with anti-PD1/PDL150,52 antibodies. Cases of subacute lupus erythematosus have been described and are the most common irAE in this category. Reports of scleroderma, dermatomyositis, and eosinophilic fasciitis have also been published.50 The average latency is 8 months (range, 0.5–26 months). Clinical features are the typical ones,50 save for the fact that scleroderma due to pembrolizumab is more diffuse and of rapid onset, while nivolumab induces a more localized reaction.52
Immunobullous EruptionsBullous pemphigoid is the main type of immunobullous eruption, although individual cases of herpetiform and linear IgA dermatoses have also been reported.11,14,56
Bullous pemphigoid, which has an incidence of 1% to 8%, has usually been linked to combined anti-PD1/PDL1 treatment, but has occasionally been associated with ipilimumab.14,19,25,56–58
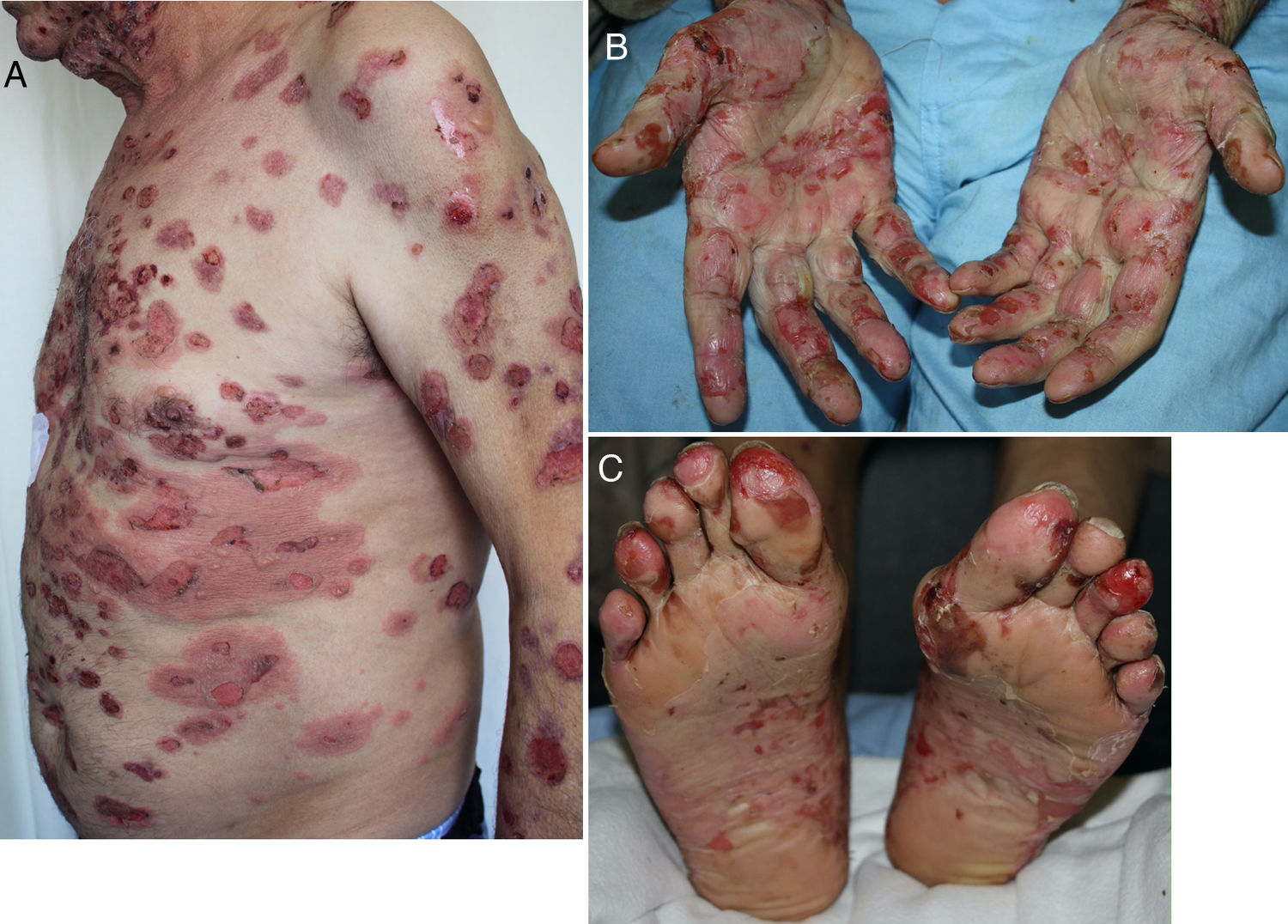
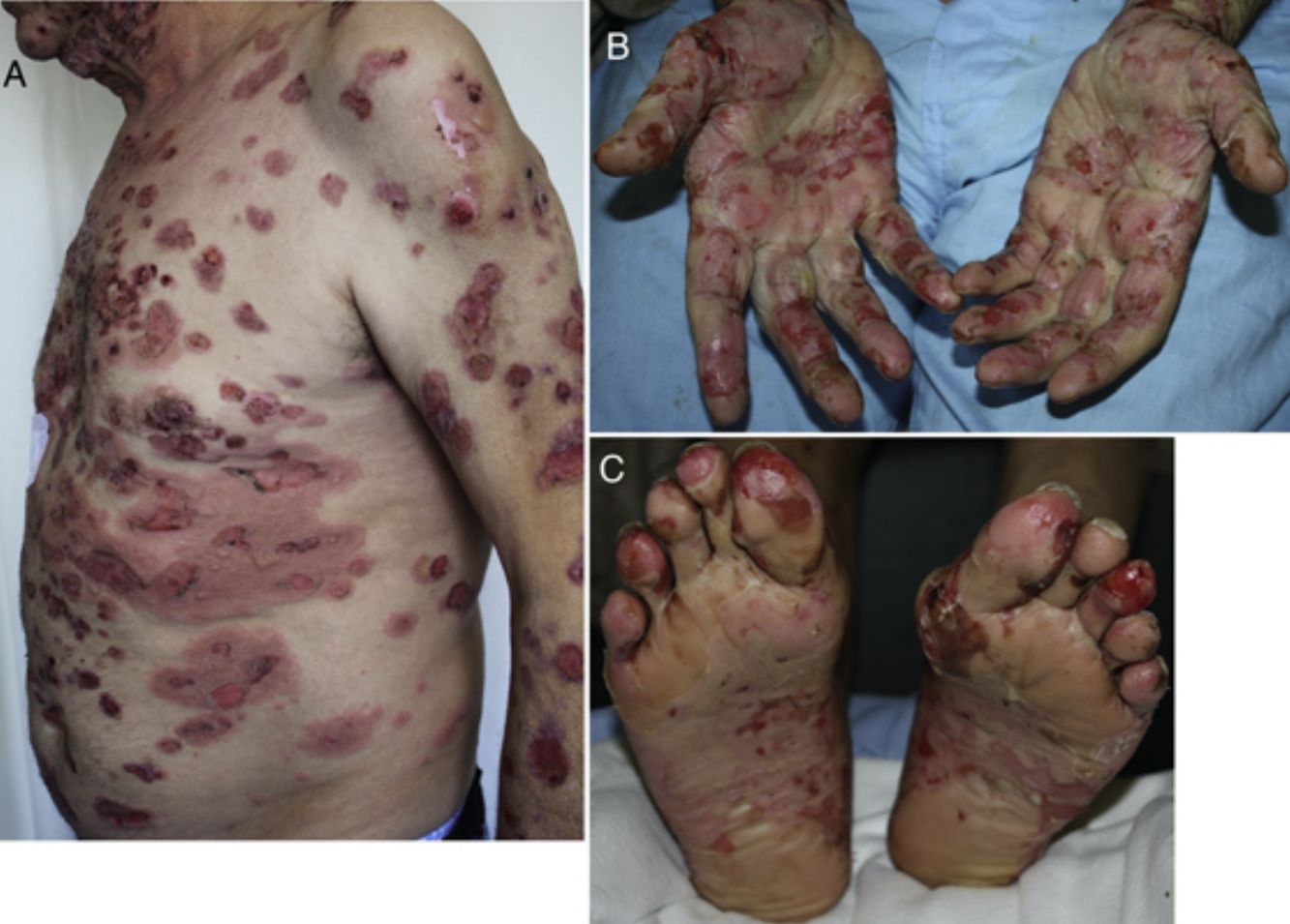
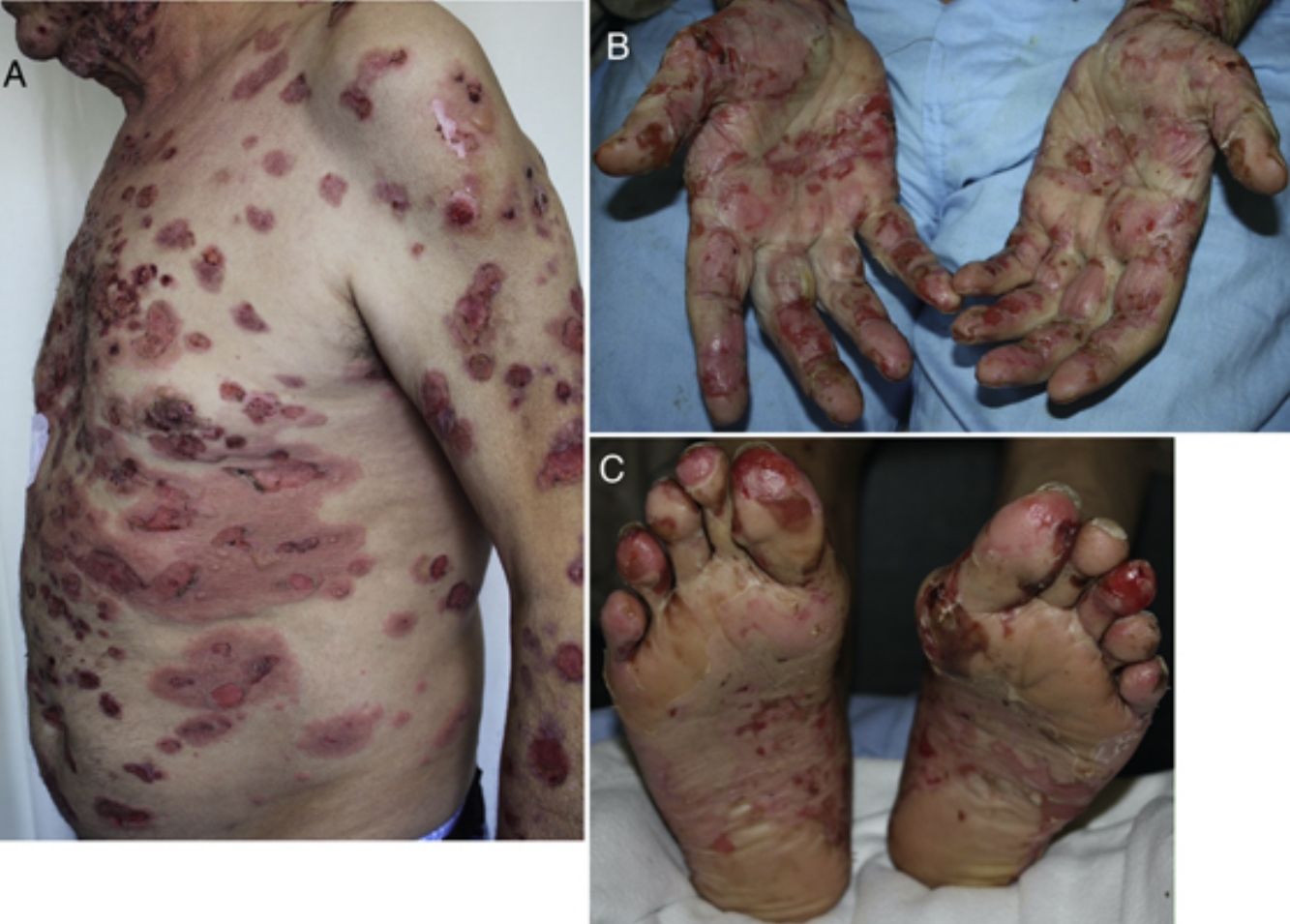
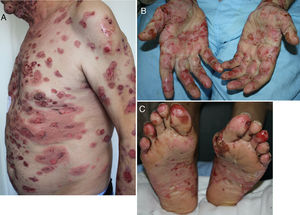
Developing 6 months after start of treatment (range, 2 weeks to 25 months),19,25,56,57,59–61 these reactions have a typical clinical picture of intense pruritus, erythema, edema, and tense blisters filled with clear fluid. They may be scattered over any part of the body (Fig. 3, A–C), localized, affect mucosal surfaces, or progress without blistering.56,60,62 There is usually a prebullous phase of a few weeks (in 34.5%) or a period with pruritus but no lesions.25,57,60,61 Subepidermal blisters rich in eosinophils are a typical finding in biopsied tissue, and direct immunofluorescence shows linear IgG and C3 deposition.20,56,60,61,63
One pathogenic mechanism proposed is cross reactivity between cutaneous and tumor antigens (as melanomas and microcytic carcinomas seem to express BP180). Another is a worsening of preexisting, subclinical pemphigoid disease due to immune system stimulus. It is unclear whether B-cell activation (caused by anti-BP180 antibodies) occurs directly on contact with ICIs or is mediated by T cells.20,62–64
For a minority of patients, symptoms can be controlled with topical corticosteroids, but more severe symptoms (grade 2 or higher) require systemic treatment. Many also require additional drugs, such as doxycycline, nicotinamide, dapsone, methotrexate, intravenous immunoglobulins, omalizumab, or rituximab. In half or more cases, ICI therapy must be suspended and systemic corticosteroids maintained, given that the clinical picture may be persistent—lasting months after the ICI is withdrawn—or recurrent.11,26,27,56,57,60,61
Some authors have reported an association between this toxicity or elevated anti-BP180 IgG titers on the one hand and a favorable response to oncologic therapy on the other.64,65
Alterations in KeratinocytesGrover disease has been reported in association with both anti-CTLA-4 and anti-PD1/PDL1 drugs.18,19 Authors recommend biopsying lesions to confirm the diagnosis, as samples show the typical signs of Grover disease.11,17,18,20
Actinic keratosis, basal cell carcinoma, seborrheic warts, epidermoid carcinoma, and eruptive keratoacanthomas have been reported in patients on anti-PD1/PDL1 blockers.14,30 The pathogenic mechanism in relation to ICIs is unknown.
Pigmentary ChangesVitiligoVitiligo is a common adverse effect linked to both anti-CTLA-4 and anti-PD1/PDL1 therapy. It develops mainly in patients with melanoma, the incidence ranging from 2.8% to 48% in case series.14,30,66,67 This irAE has also been reported in ICI-treated patients with lung cancer, however.68,69
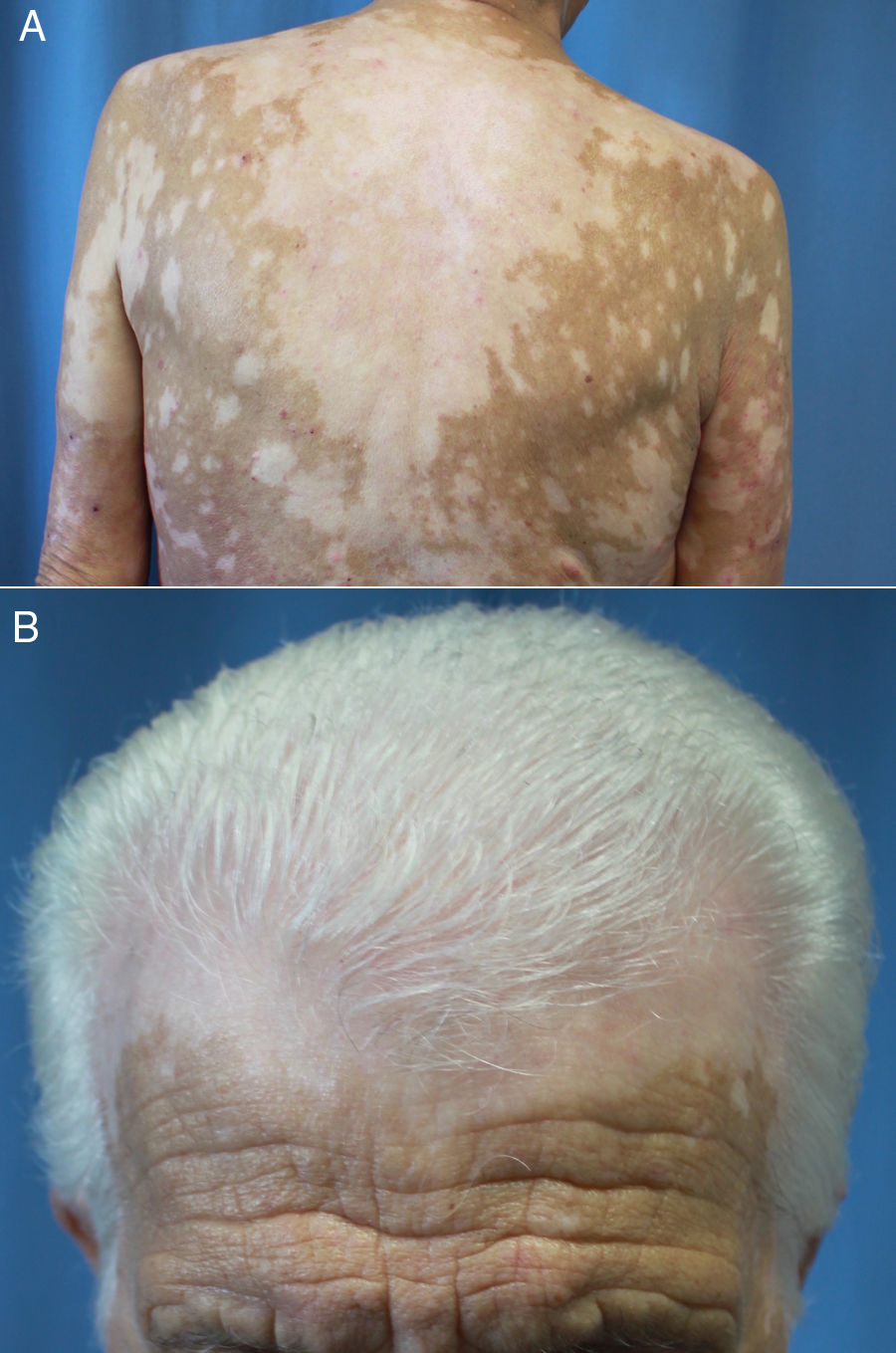
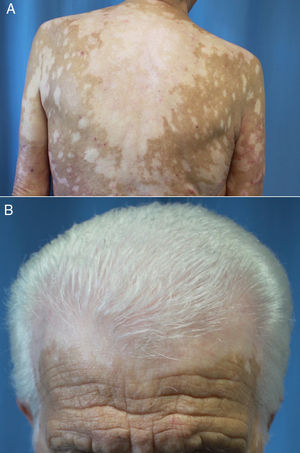
Latency after start of treatment ranges from 30 to 758 days.66,67,70,71 Reactive vitiligo differs from the common form in that it usually presents with mottled lesions that merge into larger macules distributed across sun-exposed parts of the body (Fig. 4, A and B) and is not associated with the Koebner phenomenon.66,70,72 In addition, according to Larsabal et al.,70 patients with this irAE have no family or personal histories of vitiligo, thyroiditis, or autoimmune disease but do have elevated expression of CXCR3 on CD8+ T cells in blood and perilesional tissues.70
Extensive vitiligo in a 69-year-old man treated with nivolumab for stage IV squamous cell carcinoma. The patient had no personal or family history of vitiligo. A, Confluence of mottled hypopigmented macules on the back. B, Vitiligo of the scalp associated with the whitened hair of poliosis.
The pathogenic mechanism that has been suggested is cross reactivity between tumor cells and melanocytic antigens (glycoprotein 100, MelanA/MART-1, tyrosinase, etc.).18,20,66,67
Lesions persist after ICI therapy is interrupted. Specific treatment other than protection from sun exposure is unnecessary, although topical corticosteroids, topical tacrolimus, phototherapy, and laser therapy have all been tried, with limited results.17,18,59,67,69,73
Both the appearance of vitiligo and its spread and progression have been related to a favorable response to oncologic treatment.32,66,72,74–76
Other Pigmentary AlterationsRepigmentation of gray hair and regression of preexisting melanocytic nevi or the appearance of poliosis (Fig. 3B), associated or not with vitiligo have been described in patients with ICI-treated melanoma.14,18,67,73
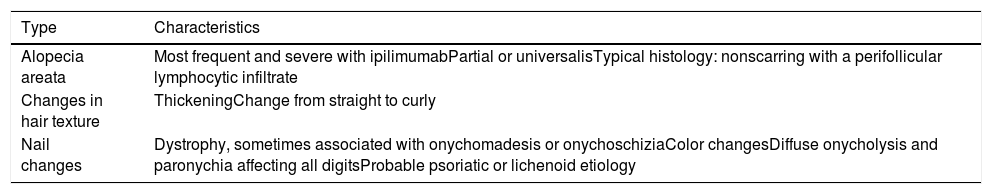
Hair and Nail AbnormalitiesThe various types of hair and nail alterations that have been reported are listed in Table 4. Hair loss—mainly alopecia areata—is the most common event, with an incidence ranging from 1% to 27% according to sources consulted.9,18
ICI-Associated Hair and Nail Alterations.
| Type | Characteristics |
|---|---|
| Alopecia areata | Most frequent and severe with ipilimumabPartial or universalisTypical histology: nonscarring with a perifollicular lymphocytic infiltrate |
| Changes in hair texture | ThickeningChange from straight to curly |
| Nail changes | Dystrophy, sometimes associated with onychomadesis or onychoschiziaColor changesDiffuse onycholysis and paronychia affecting all digitsProbable psoriatic or lichenoid etiology |
Table 5 lists other dermatoses that have been reported sporadically in single case reports. Most are inflammatory in nature. Their pathogenesis and the prognostic implications are unknown.
Other Sporadically Reported Cases of ICI-Induced Dermatoses.
| Pityriasis rubra pilaris14,19 |
| Eruptions resembling pityriasis rosea14,19 |
| Hidradenitis suppurativa87 |
| Sjögren syndrome18 |
| Acneiform eruption and papulopustular rosacea14,18 |
| Vasculitis18 |
| Erythema nodosum-type panniculitis18 |
| Acral cyanosis88 |
| Angioedema89 |
| Urticaria18 |
| Hypohidrosis90 |
| Pseudolymphoma91 |
| Granuloma annulare18 |
| Granulomatous herpes zoster92 |
| Radiation-associated dermatitis18 |
| Radiation recall dermatitis93 |
| Contact dermatitis94 |
| Pruritus simplex, nodular pruritus14 |
| Photosensitivity18 |
| Progressive mucocutaneous eruption associated with immunotherapy95 |
Abbreviation: ICI, immune checkpoint inhibitor.
Given the frequency of and morbidity associated with cutaneous irAEs, dermatology plays an important role in the multidisciplinary care of patients on ICIs. Dermatologists intervene by providing a precise diagnosis, optimal management of treatment, and a proper perspective on the prognostic relevance of skin reactions.73
Dermatologists should be involved early in the care of these patients to assess skin condition at baseline, before ICIs are introduced, or patients should at least be referred to us soon after a cutaneous toxicity appears.22,67,73 When a skin reaction presents, a detailed clinical history and an exhaustive physical examination of the skin and mucosal surfaces are necessary, and infections must be ruled out along with possible adverse effects due to other drug treatments or systemic diseases.22
The treatment of cutaneous irAEs will be based on severity, as mentioned earlier. CTCAE categories in function of BSA are currently used to classify severity.13,15,16,18 However, some authors find this grading system to be inadequate and call for evaluations based on the nature of the particular skin eruption, its location, and its effect on quality of life.17,77
Throughout the sections of this review we have pointed out that systemic corticosteroids are the cornerstone in the management of serious cutaneous toxicities (grade 2 or higher). Nonetheless, their impact on survival is a point of contention. Some studies suggest that high doses of prednisone (>10mg/d) could reduce the efficacy of ICIs and lead to a poor oncologic outcome.4,78,79 Others report that such doses do not have a negative effect on tumor response, provided the corticosteroid had not been administered before the ICI was started.17,80,81 Nonetheless, whenever possible, dermatologists should attempt to use other treatment modalities that can target the particular toxicity.17
Prognostic Significance of irAEsMore and more studies are reporting associations between cutaneous irAEs in general and certain reactions in particular on the one hand and tumor response rates on the other—as well as their association with longer progression-free and overall survival rates.4,10,32,38,64–66,71,72,74–76,82,83 However, like other authors, we believe these observations must be interpreted cautiously given the limitations of the retrospective and small-scale studies on which the conclusions are based. Moreover, the clinical significance of severe irAEs is still unclear and bias in the analysis of survival is difficult to control for: it is possible that patients who live longer also develop more irAEs simply because they have been in treatment longer.25,38,78,79,84
ConclusionsICIs are the future of oncologic therapy, and the incidence of cutaneous toxicities derived from them will rise. Although our understanding of irAEs is improving, many issues remain to be clarified regarding their characterization and classification, pathogenesis, management, and relation to prognosis. Dermatologists play an essential role in diagnosing and treating toxicities, many of which have considerable impact on cancer patients’ quality of life.
Conflict of InterestsThe authors declare that they have no conflict of interest.