Paraneoplastic pemphigus (PNP) is a rare and often fatal autoimmune mucocutaneous blistering disease that is associated with an underlying malignancy, particularly lymphoproliferative diseases. Several clinical morphologic variants of PNP have described, with the most consistent finding being severe stomatitis. Treatment of PNP is difficult and prognosis is poor.
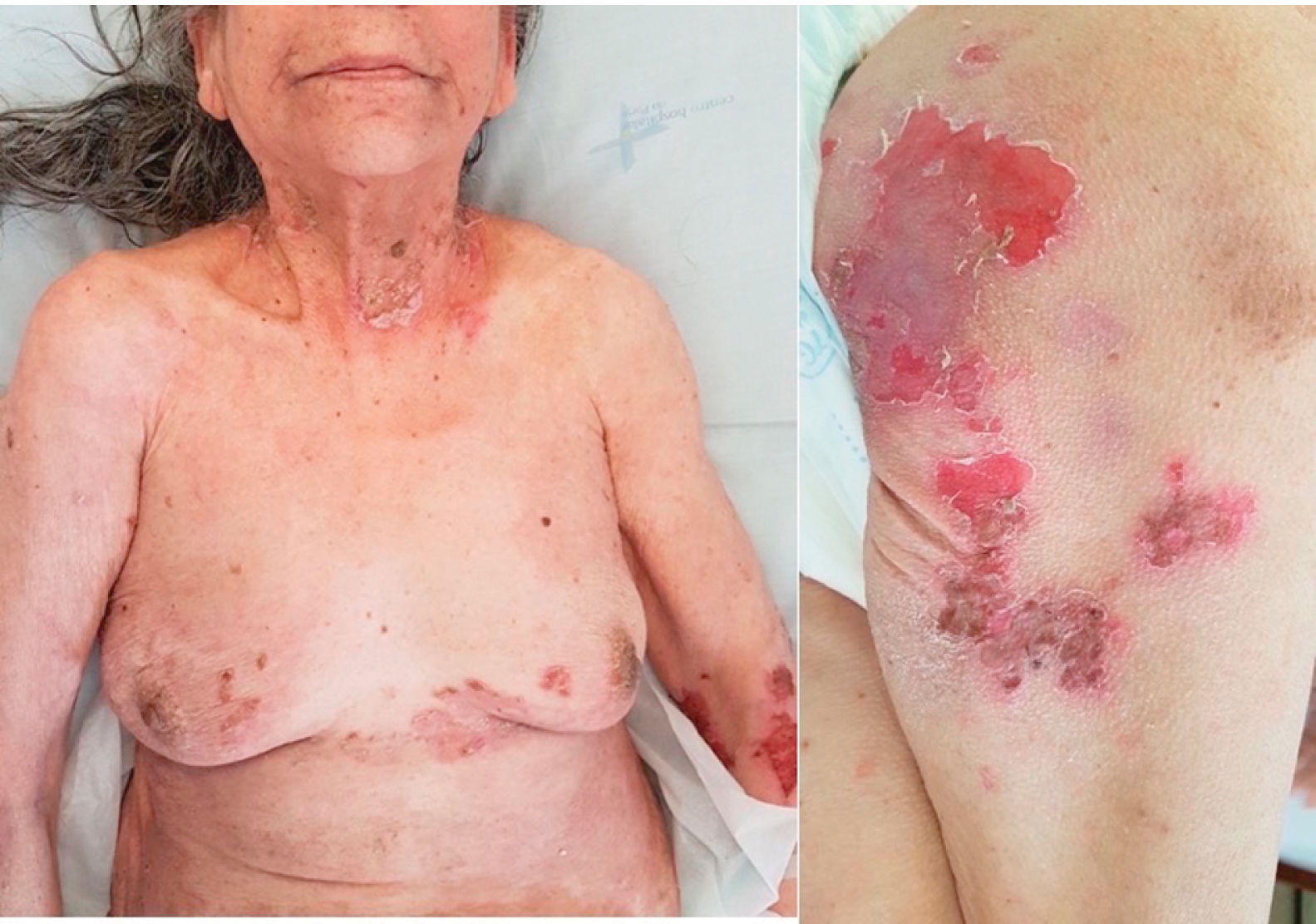
We report a case of a 79-year-old woman, with no relevant personal history, presenting with a 12-month history of pruritus and a flaccid vesiculobullous eruption. Skin examination revealed several erosions and crusts, on the trunk, arms, thighs and neck (Fig. 1). There was no involvement of the mucous membranes (oral, conjunctival or anogenital). Additionally, a hard mass with 17mm×16mm in diameter, on the right breast, was noted on physical examination.
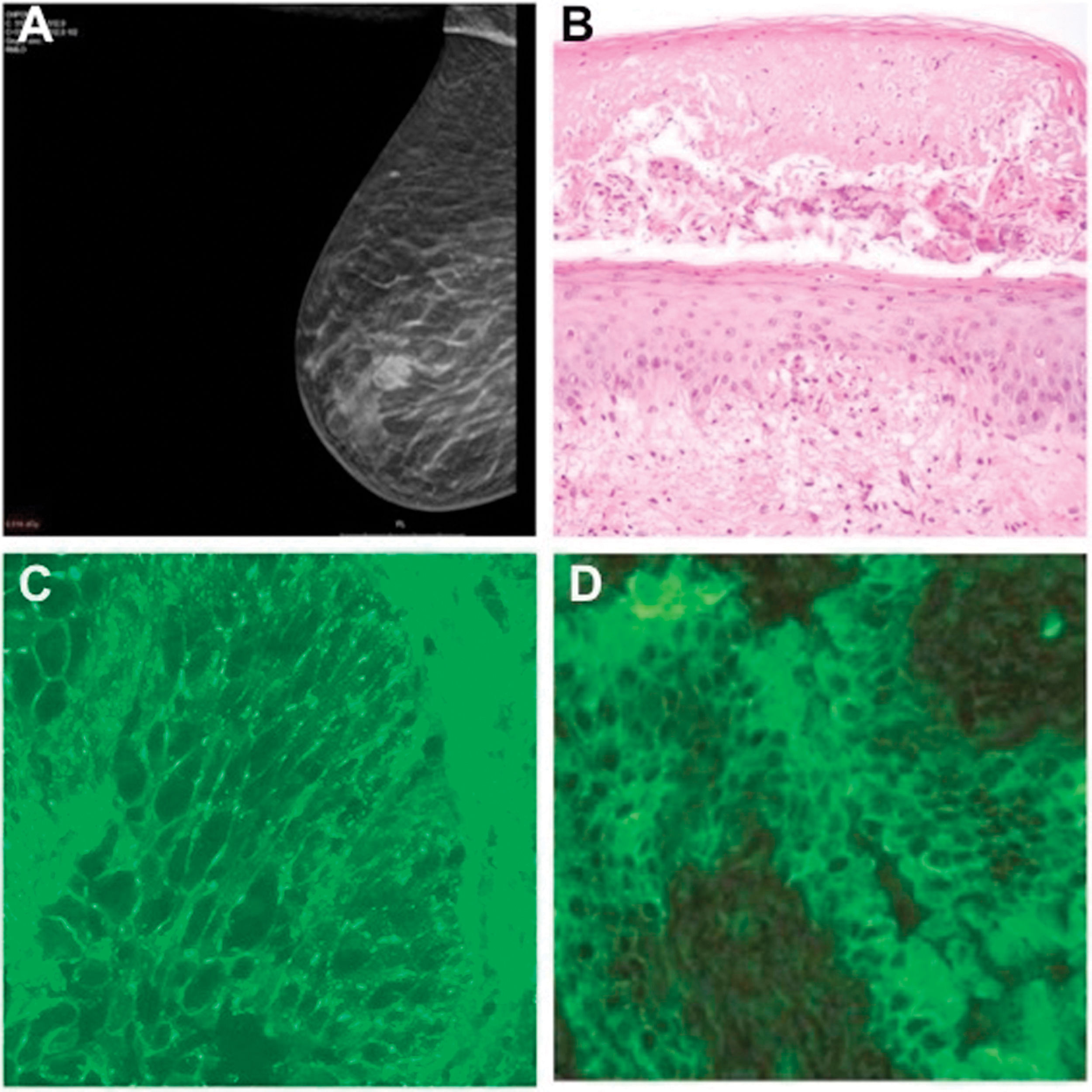
A mammography demonstrated a tumor on the right breast, which was subsequently confirmed histologically as an invasive carcinoma of no special type (Fig. 2A). Skin biopsy revealed suprabasal acantholysis and necrotic keratinocytes (Fig. 2B). Direct immunofluorescence (DIF) of perilesional skin demonstrated intercellular immunoglobulin G (IgG) deposits (Fig. 2C). Indirect immunofluorescence (IIF) on rat bladder demonstrated IgG anticell surface antibodies (Fig. 2D). Enzyme-linked immunosorbent assay (ELISA) also detected antibodies to envoplakin, and desmogleins (Dsg) 1 and 3. These results were consistent with the diagnosis of PNP.
(A) Mamography showing a tumor on the right breast. (B) Histopathology showing vesicle formation with areas of subepidermal cleft formation, suprabasilar acantholysis and necrotic keratinocytes (hematoxylin and eosin [H&E]; original magnification ×200). (C) Direct immunofluorescence of perilesional skin demonstrating intercellular immunoglobulin G staining. (D) Indirect immunofluorescence demonstrating circulating immunoglobulin G antibodies binding to rat bladder epithelium.
Skin lesions were treated with clobetasol propionate 0.05% cream with significant clinical improvement, although with cyclic relapses. The patient was referred to the Hospital's Breast Unit and started neoadjuvant hormonotherapy with anastrozole 1mg once daily. There was a good response to anastrazole, with complete resolution of skin lesions and pruritus. She refused surgical treatment and has remained lesion free for 18 months with hormonotherapy. Tumor size has also gradually reduced over time.
PNP most frequent clinical feature is severe erosive stomatitis.2 Mucosal involvement was absent in our patient, however the presence of polymorphic cutaneous lesions, in association with the results from immunofluorescence, the detection of antibodies anti-envoplakin in the patient's serum, and the concurrent breast cancer, strongly favor this diagnosis. In particular, the detection of antibodies to envoplakin is highly sensitive and specific for PNP.3
The present case represents a striking and unique form of PNP due to the absence of mucosal involvement. To our knowledge, only 2 cases have been described in the literature, one was associated with thymoma and myasthenia gravis and the other with endometrial carcinoma.4,5 The underlying cause of the absence of mucosal involvement in our patient remains unknown. Antibodies anti-Dsg 3 are thought to be important in the pathogenesis of mucosal lesions in PNP, but mucosal involvement may be seen even in those cases that are negative for anti-Dsg 3. Nevertheless, mucous membranes were spared in our patient, although circulating antibodies anti-Dsg 3 were detected.
Interestingly, PNP was induced by a breast carcinoma, which is also not common. The majority of cases of PNP are associated with hematologic malignancies, particularly non-Hodgkin lymphoma, chronic lymphocytic leukemia and Castleman's disease, however non-hematologic malignancies may occur in up to 16 percent of all cases.1,6
Diagnosis of PNP is based not only on clinical features, but also histological and immunofluorescence findings. Histological findings in PNP are variable, often resembling other disorders with similar clinical features, thus diagnosis may a true challenge.7 However, the presence of suprabasal acantholysis and keratinocytes necrosis, as observed in this patient, is a common feature. Other diagnostic criteria for PNP include DIF examination revealing cell surface and, occasionally, basement membrane zone deposits; IIF showing positive staining of rat bladder epithelium; and detection of antibodies to various proteins of the plakin gene family and Dsg 1 and Dsg 3.1,7,8 Of note, IIF is particularly useful for distinguish PNP from pemphigus vulgaris, with the latter only binding with stratified epithelium of monkey esophagus and presenting negative staining with rat bladder epithelium.9
Noteworthy, the response to treatment was better than that seen in many cases of PNP, so perhaps, this atypical presentation of PNP may be associated to a better prognosis. Further studies and follow-up are necessary to understand and elucidate the clinical importance and prognostic significance of these new findings.
In conclusion, the case of PNP present herein is unusual and unique for the absence of mucosal involvement and its association with breast cancer, and demonstrates the complexity and polymorphism of this immunobullous disease. Therefore, it is essential to recognize the importance of the correlation of clinicopathological and immunological findings in PNP and the possibility of occurring atypical presentations that should prompt immunopathologic confirmation and evaluation for an occult neoplasm.
Conflict of interestsThe authors declare that they have no conflict of interest.

![(A) Mamography showing a tumor on the right breast. (B) Histopathology showing vesicle formation with areas of subepidermal cleft formation, suprabasilar acantholysis and necrotic keratinocytes (hematoxylin and eosin [H&E]; original magnification ×200). (C) Direct immunofluorescence of perilesional skin demonstrating intercellular immunoglobulin G staining. (D) Indirect immunofluorescence demonstrating circulating immunoglobulin G antibodies binding to rat bladder epithelium. (A) Mamography showing a tumor on the right breast. (B) Histopathology showing vesicle formation with areas of subepidermal cleft formation, suprabasilar acantholysis and necrotic keratinocytes (hematoxylin and eosin [H&E]; original magnification ×200). (C) Direct immunofluorescence of perilesional skin demonstrating intercellular immunoglobulin G staining. (D) Indirect immunofluorescence demonstrating circulating immunoglobulin G antibodies binding to rat bladder epithelium.](https://static.elsevier.es/multimedia/00017310/0000011300000005/v1_202206110542/S0001731022002782/v1_202206110542/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



