Mycosis fungoides (MF) accounts for 60% of cutaneous lymphomas. Although it generally has an indolent course, 25% of cases progress to advanced stages, with a median survival of 68 months in stage IIB and 33 months in stage IVB.1 The therapeutic options for patients in these stages are limited. New molecules that have emerged in recent years include brentuximab vedotin (BV) for the treatment of CD30+ MF.2
We describe a series of 5 cases (2 women and 3 men) of advanced CD30+ MF treated with BV. The median age at diagnosis was 67 (range, 52–83) years. The cases corresponded to erythrodermic MF (1 patient), interstitial MF (1 patient), and MF with large cell transformation (3 patients).3 In all cases immunohistochemistry was positive for CD30, with expression exceeding 20% in some skin samples taken before starting treatment. The mean time since disease onset was 4.8 years. The mean number of previous treatments received by each patient was 6.6 (range, 4–9) and the median BV treatment duration was 3 cycles (range, 1–16) (Table 1).
Clinical Characteristics of Patients and Clinical Response to Treatment.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| SexClinicopathological variant | FemaleMF with LCT | MaleMF with LCT | MaleMF with LCT | FemaleErythrodermic MF | MaleInterstitial MF |
| Age | 59 | 83 | 67 | 76 | 52 |
| Prior treatments | Phototherapy, INF, PEG-INF, bexarotene, methotrexate, mogamulizumab, liposomal doxorubicin, TSEB, photopheresis | Mtx-IL, alitretinoin, acitretin, liposomal doxorubicin, CHOP, bexarotene, vorinostat, mogamulizumab, INF | PUVA, gemcitabine, TSEB, alemtuzumab, liposomal doxorubicin | PUVA, mogamulizumab, bexarotene, liposomal doxorubicin, vorinostat | acitretin, bexarotene, RT, liposomal doxorubicin |
| Clinical stage/TNMB | T4N0M0B0 IIIA | T3N0M1B2 IVB | T3N3M1B0 IVB | T4N0M0B2 IVA1 | T3NxM0B0 IIB |
| Cutaneous response7 | PR | PR | PR | DP | PR |
| Lymph node response | NA | NA | DP | NA | DP |
| Response in viscera | NA | CR | DP | NA | NA |
| Response in blood | NA | CR | NA | DP | NA |
| Overall response | PR | CR | DP | DP | DP |
| Number of BV cycles | 16 | 16 | 3 | 1 | 3 |
| Adverse effects | Paresthesia Arthralgia Myalgia | Paresthesia Asthenia Arthralgia | – | – | – |
Abbreviations: BV, brentuximab vedotin; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; DP, disease progression; INF, interferon; LCT, large cell transformation; MF, mycosis fungoides; Mtx-IL, intralesional methotrexate; NA, not applicable; PEG-INF, pegylated interferon; PUVA, psoralen-ultraviolet A; PR, partial response; RT, radiotherapy; TSEB, total skin electron beam therapy; TNMB, tumor-node-metastasis-blood.
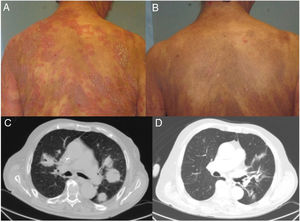
Patient 1, a 59-year-old woman with stage T4N0M0B0 MF, exhibited a clinical cutaneous response 6 weeks after starting treatment, with a response duration of 12 months (Fig. 1A and B). Patient 2, an 83-year-old man with stage T3N0M1B2 MF, showed a partial cutaneous response at 12 weeks. This patient presented CD4+/CD30+ lymphocytic infiltration in both lungs, and showed a response in viscera 15 weeks after starting treatment (Fig. 1C and D). The duration of the response in viscera was 9 months. Patient 3, a 63-year-old man with stage T3N3M1B0 MF with tumor lesions, presented a rapid cutaneous response 2 weeks after starting treatment, with subsequent progression of both lymph node and visceral disease. Patient 4, a 76-year-old woman with erythrodermic stage T4N0M0B2 MF, experienced disease progression. Patient 5, a 52-year-old man with interstitial MF with tumor lesions (stage T3NxM0B0), showed a partial cutaneous response after 2 weeks, with subsequent progression of cutaneous and lymph node disease.
Clinical and radiological response of 2 patients treated with brentuximab. A, Skin lesions in Patient 1 before starting systemic treatment. B, Clinical cutaneous response 6 weeks after starting treatment, showing a decrease in erythema and infiltration, and in the extent of the lesions. C, Axial section of contrast computed tomography scan of the chest. Tumor masses in both lung fields correspond to a proliferation of CD4+/CD30+ lymphocytes. D, Complete disappearance of the tumor masses after eighth infusion of brentuximab.
In summary, a partial cutaneous response was observed in 4 patients, of whom 2 experienced disease progression, while 1 patient showed a complete response in viscera. The median time to clinical response was 6 (range, 2–12) weeks (skin) and 15 weeks (viscera). In the 2 patients who showed a response to treatment (partial cutaneous response, complete response in viscera), without progression, the overall response duration was 9 and 12 months, respectively.
The most frequent adverse events were peripheral sensory neuropathy (maximum, grade 3), which appeared after a mean number of 2 infusions, followed by asthenia and arthralgia. None of these events necessitated treatment discontinuation. In 1 patient sensory neuropathy had resolved upon completion of BV treatment, while in the other it persisted up to 16 weeks after completion.
BV is a monoclonal antibody for CD30, a transmembrane glycoprotein expressed on activated B and T lymphocytes. This antibody is bound to monomethyl auristatin E (MMAE), an agent that disrupts the microtubule network leading to cell cycle arrest and subsequent apostosis.4 The multicenter, randomized, phase 3 ALCANZA clinical trial concluded that BV treatment was superior to both bexarotene and methotrexate, regardless of age, sex, type of cutaneous lymphoma (CD30+ MF or cutaneous anaplastic large cell lymphoma), or involvement (cutaneous or cutaneous and visceral).5 However, up to a third of the patients had early-stage disease and a median of only 2 previous lines of treatment, thereby excluding, in our opinion, the majority of patients who receive this type of treatment in routine clinical practice. Papadavid et al. have shared their experience with BV treatment in 3 patients with folliculotropic MF and large cell transformation and 1 patient with Sézary syndrome (SS).6 The 3 MF patients showed complete or partial responses, while the SS patient presented stable disease.6,7
The most surprising finding in our case series was the rapid cutaneous response after the first infusion. This was particularly evident in the case of tumor lesions, which had been rapidly progressing to skin necrosis. Patient 5, who had a diagnosis of interstitial MF and tumor-like skin lesions, experienced this effect after a single dose of BV. Another interesting finding was the complete response in viscera, according to the relevant criterion, which was observed in a patient with specific pulmonary involvement and was maintained for 9 months.
In our experience BV offers a rapid tumor response, although maintenance of this response is highly variable. Knowledge of the type of patient and the disease stage is necessary to ensure optimal use. Furthermore, new studies are required to best position the drug in clinical practice, including its combination with other agents and maintenance therapy in cases in which a response is achieved.
Conflicts of InterestThe authors declare that they have no conflicts of interest.