The administration of appropriate doses of active ingredients and excipients is crucial for achieving desired treatment outcomes in pediatric dermatology. A number of factors need to be considered, including the characteristics of the lesion, the patient, and the drug. An additional challenge in pediatric settings is the limited number of commercially available formulations suitable for use in children. Drug compounding, which is the preparation of medications tailored to the needs of individual patients, is a good alternative for pediatric populations for a number of reasons. Using a customized compound, the clinician can prescribe formulations that contain the optimal dose of the active ingredients within acceptable limits and the most suitable vehicle and formulation components. Compounding can also be used to combine several active ingredients in a single medication and even adapt the vehicle to the characteristics of the lesion and the needs of the patient. The pharmaceutical formulations described in this review are based on extensive clinical experience and can be customized to meet individual needs.
En dermatología pediátrica, la correcta elección de la dosis de principio activo así como del excipiente, se vuelve fundamental para conseguir los resultados terapéuticos deseados, por lo que se deben tener en cuenta aspectos tan variados como las características de la lesión, las del paciente, así como las del medicamento seleccionado. En la población pediátrica, se plantean dificultades añadidas tales como la limitada variedad de especialidades comerciales que se ajusten a sus particularidades. Por ello, la formulación magistral o formulación de medicamentos individualizados supone una buena alternativa terapéutica que permite emplear principios activos en los rangos terapéuticos aceptados, vehiculizados en las formas farmacéuticas idóneas, asociar varios en un mismo medicamento e incluso adaptar el vehículo al estado de la lesión, así como a las necesidades intrínsecas del paciente.Las formulaciones recogidas en este artículo, se basan en una amplia experiencia clínica y permiten a los médicos prescriptores adaptar el tratamiento de forma personalizada.
The skin of infants and children is characterized by being softer than that of adults. In infants, the stratum corneum is thinner and transepidermal water loss (TEWL) is greater than in 5-year-old children or in adults. The skin’s natural hydration and lipid production are lower than in 5-year-old children, in whom these factors are similar to those of adults.1
Given these differences in development, the skin of children is more susceptible to irritation and inflammation in the face of certain aggressions than that of adults. TEWL measured on the cheek is greater in 1-year-old children than in 5-year-old children or in adults.2
Small children, and especially infants, can therefore absorb a greater amount of topically applied substances than adults, and they also have a reduced ability to metabolize and excrete any given substance.3
This means that children have a greater risk of suffering from adverse effects and toxicity due to topical drugs than adults. Moreover, children have a greater risk of systemic toxicity from topical drugs given the higher ratio between body surface area and weight. Thus, application of the same amount will produce a higher systemic concentration (mg/kg) in children than in adults.4
According to the World Health Organization (WHO), more than half of children in developed nations receive drugs whose doses are designed for adults and are not authorized for use in minors.5
The lack of pharmaceutical preparations aimed at the treatment of skin diseases in children is notable. A large percentage of drugs used in these cases is estimated to be indicated for use in the adult population only.
This means that pharmaceutical formulations are largely being used that do not have sufficient information regarding their use, highlighting the need to use the formulation as a top-level therapeutic tool for achieving optimum safety and efficacy criteria.
Following is a series of proposed formulations for the most common pediatric skin diseases or for those for which no commercially available therapeutic alternative exists. These formulas are shown at the most common concentration, indicating the dose ranges in 2 age ranges (from 0 to 2 years and from 3 to 12 years) and specifying the ideal vehicle for each of them.
It should be noted that the main problem with topical treatment with salicylic acid is the possibility of percutaneous absorption, causing salicylate poisoning with symptoms such as burning in the oral mucosa, frontal lobe headache, metabolic acidosis, central nervous system symptoms, tinnitus, nausea, and vomiting. The risk is greater in the treatment of large body surface areas and in children. It should therefore not be used on more than 10% of body surface in children. In general, cases of poisoning due to topical application of salicylates have been in children under the age of 3 years.6
Atopic DermatitisThe formulations proposed for the treatment of this disease are classified according to the phase of the lesion, thus adapting the vehicle for the active ingredients to the characteristics of the area to be treated. Topical treatment continues to be a basic pillar in the approach to atopic dermatitis.
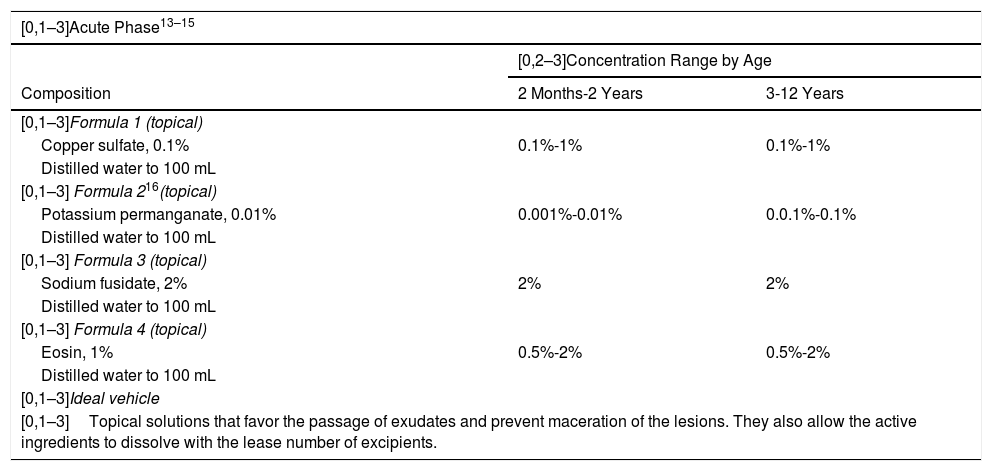
Acute PhaseThe objective is to control the exudation of the acute lesions by using antimicrobial and astringent active ingredients, such as copper sulfate, potassium permanganate, or eosin (Table 1).7
Proposed Formulas for the Treatment of Atopic Dermatitis in Children.
| [0,1–3]Acute Phase13–15 | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| [0,1–3]Formula 1 (topical) | ||
| Copper sulfate, 0.1% | 0.1%-1% | 0.1%-1% |
| Distilled water to 100 mL | ||
| [0,1–3] Formula 216(topical) | ||
| Potassium permanganate, 0.01% | 0.001%-0.01% | 0.0.1%-0.1% |
| Distilled water to 100 mL | ||
| [0,1–3] Formula 3 (topical) | ||
| Sodium fusidate, 2% | 2% | 2% |
| Distilled water to 100 mL | ||
| [0,1–3] Formula 4 (topical) | ||
| Eosin, 1% | 0.5%-2% | 0.5%-2% |
| Distilled water to 100 mL | ||
| [0,1–3]Ideal vehicle | ||
| [0,1–3]Topical solutions that favor the passage of exudates and prevent maceration of the lesions. They also allow the active ingredients to dissolve with the lease number of excipients. | ||
| [0,1–3]Subacute Phase13,14 | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| [0,1–3]Formula 1 (topical) | ||
| Hydrocortisone, 1% | 0.5%-2.5% | 0.5%-2.5% |
| Gentamicin, 0.1% | 0.05%-0.1% | 0.05%-0.1% |
| Oatmeal extract, 5% | 5% | 5% |
| Aloe vera, 5% | 5%-10% | 5%-10% |
| Oil-water emulsion to 100 g | ||
| [0,1–3]Formula 2 (topical) | ||
| Prednicarbate, 0.1% | Noa | 0.1%-0.25% |
| Sodium fusidate, 2% | 2% | 2% |
| Extract of Centella asiatica, 1% | 1% | 1% |
| Allantoin, 1% | 1%-2% | 1%-2% |
| Oil-water lotion to 200 g | ||
| [0,1–3]Evidence exists regarding the efficacy of using prednicarbate, 1% in patients from 4 months of age for 3 weeks of treatment.17 | ||
| [0,1–3]Formula 3 (topical) | ||
| Betamethasone valerate, 0.05% | Noa | 0.01%-0.2% |
| Not facial/Not in skin folds | ||
| Ichthyol, 1% | Not recommended | 0.5%-2% |
| Water-based paste to 100 g | ||
| [0,1–3]Ideal vehicle | ||
| [0,1–3]Certain fat content. Considering the state of the lesion, we suggest formulations in oil-water emulsion, oil-water lotion, and water-based paste (ordered by percentage oil phase, from greater to lesser), depending on the level of occlusion desired | ||
| [0,1–3]Chronic Phase13–15 | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| [0,1–3]Formula 1 (topical) | ||
| Triamcinolone acetonide, 0.05% | No | 0.025%-0.1%b |
| Not facial/Not in skin folds | ||
| Urea, 10% | 10%-20% | 10%-20% |
| Lanolin, 5% | 5%-10% | 5%-10% |
| Oil-water emulsion to 100 g | ||
| [0,1–3]Formula 2 (topical) | ||
| Prednicarbate, 0.1% | Noc | 0.1%-0.25% |
| Salicylic acid, 5% | No | Max, 10% |
| Urea, 10% | 10%-20% | 10%-20% |
| Tacrolimus, 0.03% | Nod | 0.03% |
| Allantoin, 1% | 1%-2% | 1%-2% |
| Rosehip oil, 5% | 5%-20% | 5%-20% |
| Hydrophilic ointment to 100 g | ||
| [0,1–3]Ideal vehicle | ||
| [0,1–3]Greater degree of occlusion, such as petrolatum or ointment. Nevertheless, several alternatives are proposed so that the prescribing physician can decide which is more appropriate for the patient. All of them allow for greater hydration and regeneration of the lesion, as this phase involves lichenification and a high degree of xerosis. | ||
Evidence exists on the efficacy of prednicarbate, 0.1% in patients aged 4 months and over for 3 weeks of treatment.17.
The objective is to control the inflammation, pruritus, and possible superinfection of the lesions. Corticosteroids of different potencies are used (from lower to higher, hydrocortisone, prednicarbate, and betamethasone) for the inflammation and pruritus.8 Other anti-inflammatory substances such as aloe vera, oatmeal extract, or allantoin are used for their anti-inflammatory, re-epithelizing, and calming properties.9,10
The addition of mild tars such, as ichthyol, can reduce the reactivity of the skin, thus reducing pruritus, erythema, and inflammation. It should always be remembered that the use of coal tars is associated with increased risk of cancer and they should therefore only be used for short periods of time and over small areas and should preferably be reserved for older children.
Current guidelines do not recommend the use of topical products that combine antibiotics with corticosteroids.11 Exceptionally, antibiotics (gentamicin, sodium fusidate) may be combined with topical corticosteroids for the treatment of specific eczema plaques with confirmed infection in order to avoid the use of systemic antibiotics and to favor adherence to treatment (only 2 topical applications per day instead of 3 or 4) (Table 1).12
Chronic PhaseLichenification of the lesions is added to the inflammation in the chronic phase. As well as the classic anti-inflammatory active ingredients, such as corticosteroids and calcineurin inhibitors, allantoin or rosehip oil may be used as coadjuvant treatment due to their anti-inflammatory, calming, and antioxidant properties.9 Urea and salicylic acid will help to control lichenified lesions. Application of salicylic acid should always be carried out with caution, as it may cause metabolic acidosis. It is therefore not recommended in children under 2 years of age and its use should be limited in terms of the surface area to be treated (< 10% of skin area) and duration of treatment (Table 1).
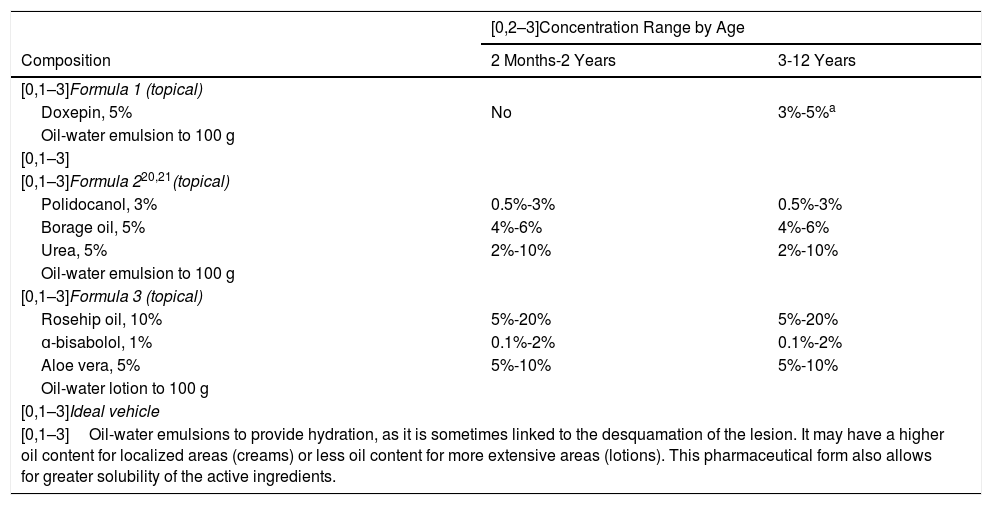
PruritusThe formulations proposed in this section are mainly aimed at alleviating intense pruritus that affects the patient’s quality of life. The active ingredients of choice are anti-inflammatory and analgesic agents, and, for more severe cases, local anesthetics are proposed (Table 2).
Proposed Formulas for the Management of Pruritus in Children.13,14.
| [0,2–3]Concentration Range by Age | ||
|---|---|---|
| Composition | 2 Months-2 Years | 3-12 Years |
| [0,1–3]Formula 1 (topical) | ||
| Doxepin, 5% | No | 3%-5%a |
| Oil-water emulsion to 100 g | ||
| [0,1–3] | ||
| [0,1–3]Formula 220,21(topical) | ||
| Polidocanol, 3% | 0.5%-3% | 0.5%-3% |
| Borage oil, 5% | 4%-6% | 4%-6% |
| Urea, 5% | 2%-10% | 2%-10% |
| Oil-water emulsion to 100 g | ||
| [0,1–3]Formula 3 (topical) | ||
| Rosehip oil, 10% | 5%-20% | 5%-20% |
| ɑ-bisabolol, 1% | 0.1%-2% | 0.1%-2% |
| Aloe vera, 5% | 5%-10% | 5%-10% |
| Oil-water lotion to 100 g | ||
| [0,1–3]Ideal vehicle | ||
| [0,1–3]Oil-water emulsions to provide hydration, as it is sometimes linked to the desquamation of the lesion. It may have a higher oil content for localized areas (creams) or less oil content for more extensive areas (lotions). This pharmaceutical form also allows for greater solubility of the active ingredients. | ||
We have avoided proposing local anesthetics, as they can cause methemoglobinemia in children. They should be used in small quantities and avoided whenever possible.19
Seborrheic DermatitisTopical treatment continues to be a basic pillar in the treatment of seborrheic dermatitis.
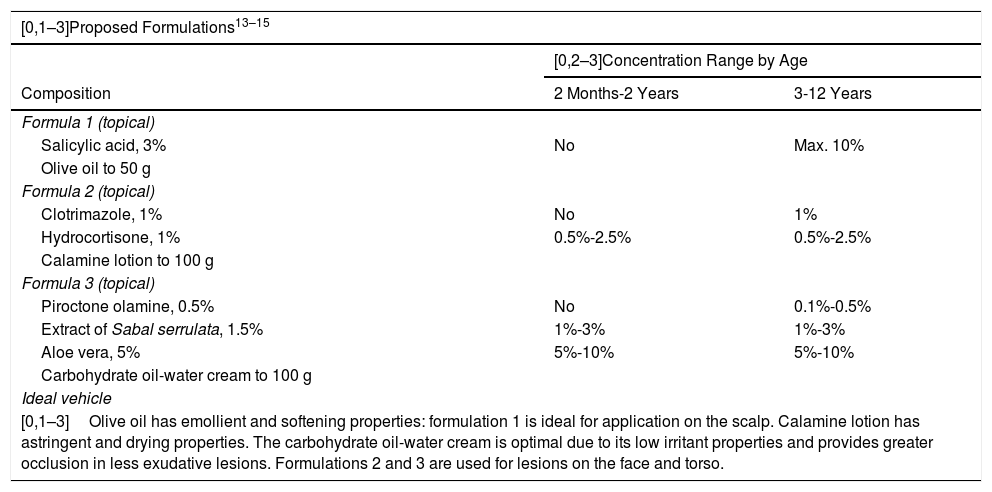
The objective of the proposed formulations is to eliminate the scales of the lesions by means of a keratolytic agent (salicylic acid), antifungal agents (clotrimazole or piroctone olamine), and anti-inflammatory agents (hydrocortisone, aloe vera).22
Extract of Sabal serrulata is an antiseborrheic agent that acts directly on the mechanisms that produce sebum, which is why it is therapeutically useful in this case.23
As noted above, application of salicylic acid should always be carried out with caution, as it may cause metabolic acidosis. It is not recommended in children under 2 years of age and its use should be limited in terms of the skin surface area to be treated (< 10%) and duration of treatment in older children.
The active ingredients should be used in a moisturizing vehicle with occlusive power that favors regeneration of the skin’s protective barrier and controls excessive growth of Malassezia spp (Table 3).
Proposed Formulas for the Treatment of Seborrheic Dermatitis in Children.
| [0,1–3]Proposed Formulations13–15 | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Salicylic acid, 3% | No | Max. 10% |
| Olive oil to 50 g | ||
| Formula 2 (topical) | ||
| Clotrimazole, 1% | No | 1% |
| Hydrocortisone, 1% | 0.5%-2.5% | 0.5%-2.5% |
| Calamine lotion to 100 g | ||
| Formula 3 (topical) | ||
| Piroctone olamine, 0.5% | No | 0.1%-0.5% |
| Extract of Sabal serrulata, 1.5% | 1%-3% | 1%-3% |
| Aloe vera, 5% | 5%-10% | 5%-10% |
| Carbohydrate oil-water cream to 100 g | ||
| Ideal vehicle | ||
| [0,1–3]Olive oil has emollient and softening properties: formulation 1 is ideal for application on the scalp. Calamine lotion has astringent and drying properties. The carbohydrate oil-water cream is optimal due to its low irritant properties and provides greater occlusion in less exudative lesions. Formulations 2 and 3 are used for lesions on the face and torso. | ||
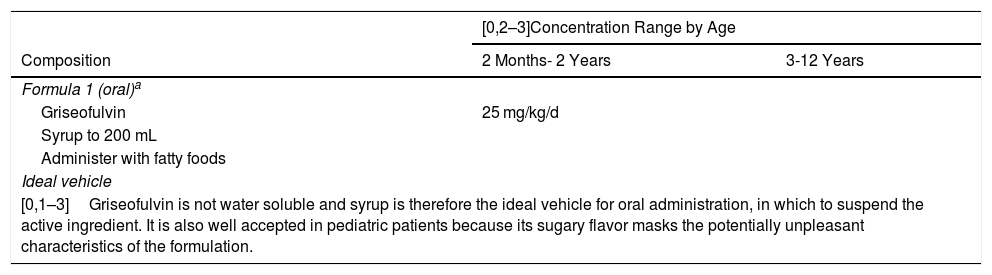
The treatment of tinea capitis must always be systemic. The antifungal agent griseofulvin is the ingredient of choice, especially in tinea caused by the genus Microsporum (Table 4).24 The appropriate dosage of griseofulvin is 25 mg/kg/d.25
Proposed Formulas for the Treatment of Tinea Capitis.13,14.
| [0,2–3]Concentration Range by Age | ||
|---|---|---|
| Composition | 2 Months- 2 Years | 3-12 Years |
| Formula 1 (oral)a | ||
| Griseofulvin | 25 mg/kg/d | |
| Syrup to 200 mL | ||
| Administer with fatty foods | ||
| Ideal vehicle | ||
| [0,1–3]Griseofulvin is not water soluble and syrup is therefore the ideal vehicle for oral administration, in which to suspend the active ingredient. It is also well accepted in pediatric patients because its sugary flavor masks the potentially unpleasant characteristics of the formulation. | ||
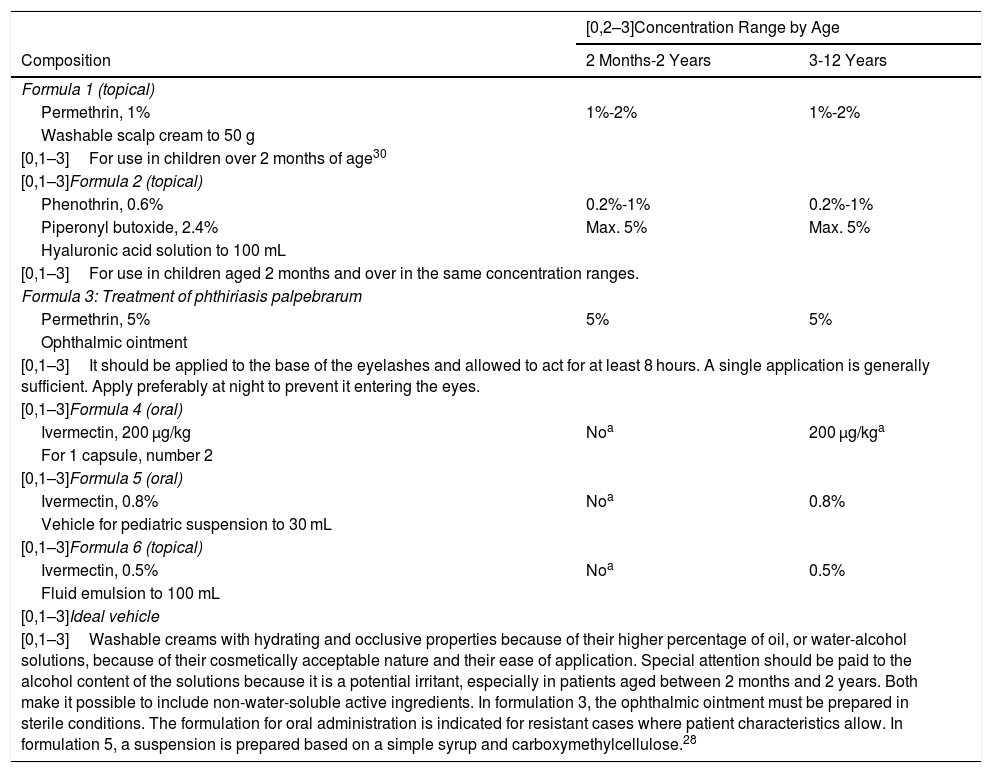
Table 5 shows the proposed combinations for the treatment of pediculosis, including active ingredients with insecticidal and antiparasitic properties, applied topically, with the objective of eliminating the lice. In cases of pediculosis resistant to topical treatment, oral ivermectin (formulations 4 and 5)28 and topical ivermectin (formulation 6) may be used.
Proposed Formulas for the Treatment of Pediculosis in Children.13,14.
| [0,2–3]Concentration Range by Age | ||
|---|---|---|
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Permethrin, 1% | 1%-2% | 1%-2% |
| Washable scalp cream to 50 g | ||
| [0,1–3]For use in children over 2 months of age30 | ||
| [0,1–3]Formula 2 (topical) | ||
| Phenothrin, 0.6% | 0.2%-1% | 0.2%-1% |
| Piperonyl butoxide, 2.4% | Max. 5% | Max. 5% |
| Hyaluronic acid solution to 100 mL | ||
| [0,1–3]For use in children aged 2 months and over in the same concentration ranges. | ||
| Formula 3: Treatment of phthiriasis palpebrarum | ||
| Permethrin, 5% | 5% | 5% |
| Ophthalmic ointment | ||
| [0,1–3]It should be applied to the base of the eyelashes and allowed to act for at least 8 hours. A single application is generally sufficient. Apply preferably at night to prevent it entering the eyes. | ||
| [0,1–3]Formula 4 (oral) | ||
| Ivermectin, 200 μg/kg | Noa | 200 μg/kga |
| For 1 capsule, number 2 | ||
| [0,1–3]Formula 5 (oral) | ||
| Ivermectin, 0.8% | Noa | 0.8% |
| Vehicle for pediatric suspension to 30 mL | ||
| [0,1–3]Formula 6 (topical) | ||
| Ivermectin, 0.5% | Noa | 0.5% |
| Fluid emulsion to 100 mL | ||
| [0,1–3]Ideal vehicle | ||
| [0,1–3]Washable creams with hydrating and occlusive properties because of their higher percentage of oil, or water-alcohol solutions, because of their cosmetically acceptable nature and their ease of application. Special attention should be paid to the alcohol content of the solutions because it is a potential irritant, especially in patients aged between 2 months and 2 years. Both make it possible to include non-water-soluble active ingredients. In formulation 3, the ophthalmic ointment must be prepared in sterile conditions. The formulation for oral administration is indicated for resistant cases where patient characteristics allow. In formulation 5, a suspension is prepared based on a simple syrup and carboxymethylcellulose.28 | ||
aOver 5 years of age or more than 15 kg in weight. However, its use off indication in children weighing under 15 kg has been shown to be safe and effective, with mild adverse effects presenting in a small group of patients.31 It should be borne in mind that children under the age of 6 years are understood not to be able to swallow tablets.28.
Ivermectin can be prepared as capsules for young patients who can swallow them, but also as a 0.8% oral suspension, which allows for a convenient dosage of 1 drop per kilogram of bodyweight, which makes it easy to use in small children and infants.28.
The formulation for oral administration is indicated only for resistant cases.
The activities of these active ingredients consist of their action on the parasite’s nerve cell membranes, leading to its paralysis and death. These formulations make it possible to adjust the dose based on the age of the patient and constitute a very interesting alternative to conventional commercially available treatments.
We also include a formula (formulation 3) for the treatment of pediatric pediculosis corporis on the eyelashes, which is the only alternative treatment option.
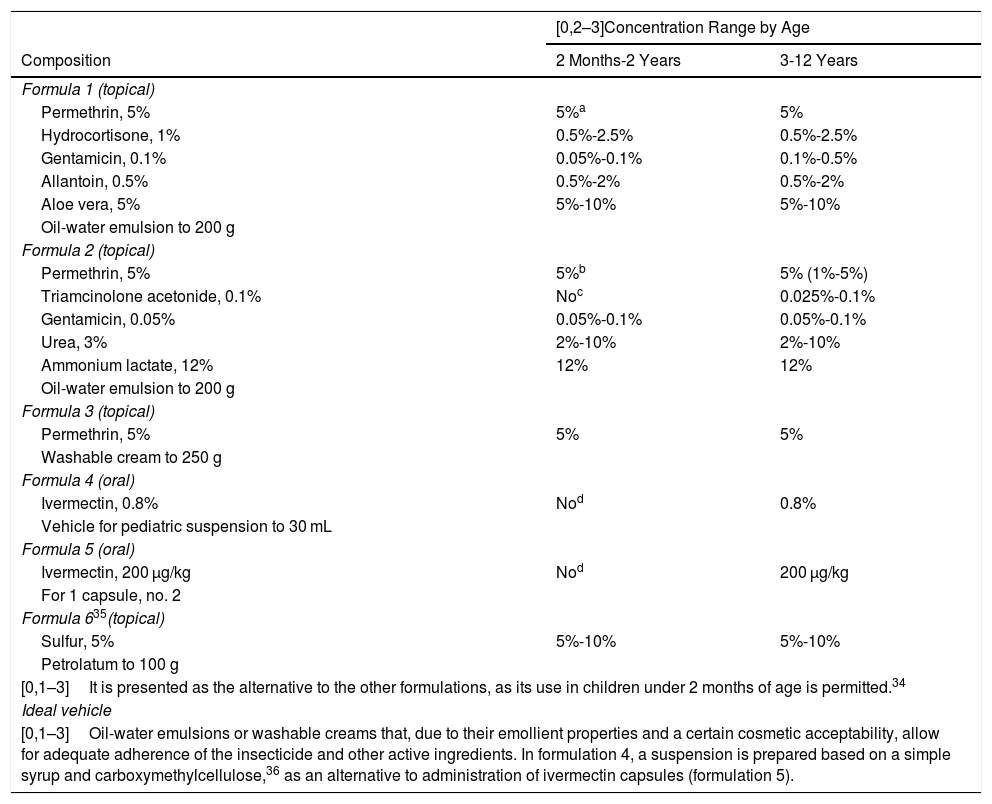
ScabiesActive ingredients with insecticidal and antiparasitic properties applied topically with the objective of the rapid elimination of the infestation caused by the mite in refractory cases.32 In most cases, patients present eczematous lesions secondary to scratching and the irritation caused by the acaricides. Table 6 shows some proposed combinations that may be of interest in managing these cases.33
Proposed Formulas for the Treatment of Scabies.13–15.
| [0,2–3]Concentration Range by Age | ||
|---|---|---|
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Permethrin, 5% | 5%a | 5% |
| Hydrocortisone, 1% | 0.5%-2.5% | 0.5%-2.5% |
| Gentamicin, 0.1% | 0.05%-0.1% | 0.1%-0.5% |
| Allantoin, 0.5% | 0.5%-2% | 0.5%-2% |
| Aloe vera, 5% | 5%-10% | 5%-10% |
| Oil-water emulsion to 200 g | ||
| Formula 2 (topical) | ||
| Permethrin, 5% | 5%b | 5% (1%-5%) |
| Triamcinolone acetonide, 0.1% | Noc | 0.025%-0.1% |
| Gentamicin, 0.05% | 0.05%-0.1% | 0.05%-0.1% |
| Urea, 3% | 2%-10% | 2%-10% |
| Ammonium lactate, 12% | 12% | 12% |
| Oil-water emulsion to 200 g | ||
| Formula 3 (topical) | ||
| Permethrin, 5% | 5% | 5% |
| Washable cream to 250 g | ||
| Formula 4 (oral) | ||
| Ivermectin, 0.8% | Nod | 0.8% |
| Vehicle for pediatric suspension to 30 mL | ||
| Formula 5 (oral) | ||
| Ivermectin, 200 μg/kg | Nod | 200 μg/kg |
| For 1 capsule, no. 2 | ||
| Formula 635(topical) | ||
| Sulfur, 5% | 5%-10% | 5%-10% |
| Petrolatum to 100 g | ||
| [0,1–3]It is presented as the alternative to the other formulations, as its use in children under 2 months of age is permitted.34 | ||
| Ideal vehicle | ||
| [0,1–3]Oil-water emulsions or washable creams that, due to their emollient properties and a certain cosmetic acceptability, allow for adequate adherence of the insecticide and other active ingredients. In formulation 4, a suspension is prepared based on a simple syrup and carboxymethylcellulose,36 as an alternative to administration of ivermectin capsules (formulation 5). | ||
aFor use in children aged 2 months and over. Formulation 6 in this section is proposed as an alternative for this age.26.
bFor use in children aged 2 months and over.
cFor use in children aged 3 years and over.
dMore than 5 years of age or 15 kg in bodyweight. However, its use off indication in children weighing under 15 kg has been shown to be safe and effective, with mild adverse effects presenting in a small group of patients.31 It should be borne in mind that children under the age of 6 years are understood not to be able to swallow tablets.20.
Ivermectin can be prepared as capsules for young patients who can swallow them, but also as a 0.8% oral suspension, which allows for a convenient dosage of 1 drop per kilogram of bodyweight, which makes it easy to use in small children and infants.28.
The formulation for oral administration is indicated only for resistant cases.
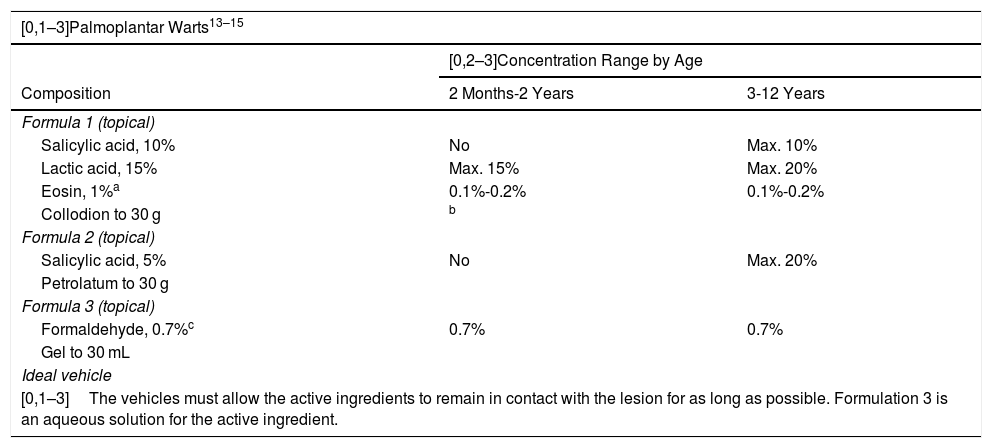
The formulations for the treatment of warts vary depending on the type and number of warts and their location and size, and the age of the patient.36 Many alternatives exist for the treatment of warts using formulations with a single active ingredient or a combination of ingredients (Table 7). The principal objective of the treatment consists of destroying and reducing the proliferation of the infected epithelium.37
Proposed Formulas for the Treatment of Warts in Children.
| [0,1–3]Palmoplantar Warts13–15 | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Salicylic acid, 10% | No | Max. 10% |
| Lactic acid, 15% | Max. 15% | Max. 20% |
| Eosin, 1%a | 0.1%-0.2% | 0.1%-0.2% |
| Collodion to 30 g | b | |
| Formula 2 (topical) | ||
| Salicylic acid, 5% | No | Max. 20% |
| Petrolatum to 30 g | ||
| Formula 3 (topical) | ||
| Formaldehyde, 0.7%c | 0.7% | 0.7% |
| Gel to 30 mL | ||
| Ideal vehicle | ||
| [0,1–3]The vehicles must allow the active ingredients to remain in contact with the lesion for as long as possible. Formulation 3 is an aqueous solution for the active ingredient. | ||
| [0,1–3]Flat Warts | ||
|---|---|---|
| Composition | [0,2–3]Concentration Range by Age | |
| 2 Months-2 Years | 3-12 Years | |
| Formula 1 (topical) | ||
| Retinoic acid, 0.02% | 0.02%-0.05%d | 0.02%-0.05%d |
| Salicylic acid, 3% | No | Max. 10%e |
| Hydrocortisone, 1% | 0.5%-2.5% | 0.5%-2.5% |
| Oil-water cream to 50 g | ||
| Ideal vehicle | ||
| [0,1–3]Ideal vehicle with medium oil content (approximately 20%-30%) for carrying the active ingredients is a Beeler base cream because of its oil-water makeup, mostly applied to the face and front surface of the legs. | ||
| [0,1–3]Common Warts | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Salicylic acid, 8% | No | Max. 10% |
| Lactic acid, 8% | Max. 15% | Max. 20% |
| Eosin, 0.1% | 0.1%-0.2% | 0.1%-0.2% |
| Collodion to 30 g | f | |
| Formula 2 (topical) | ||
| Salicylic acid, 5% | No | Max. 10% |
| Petrolatum to 30 g | ||
| Ideal vehicle | ||
| [0,1–3]The collodion makes it possible to dissolve the components in a liquid vehicle that, when it solidifies, generates prolonged permanence of the drug. The petrolatum ensures good adhesion to the area of the lesion. | ||
Because of its high concentration of solvents, collodion may be an irritant in children under 2 years of age.
Topical use of retinoic acid has been shown to be effective in treatments of 6 weeks and longer.38.
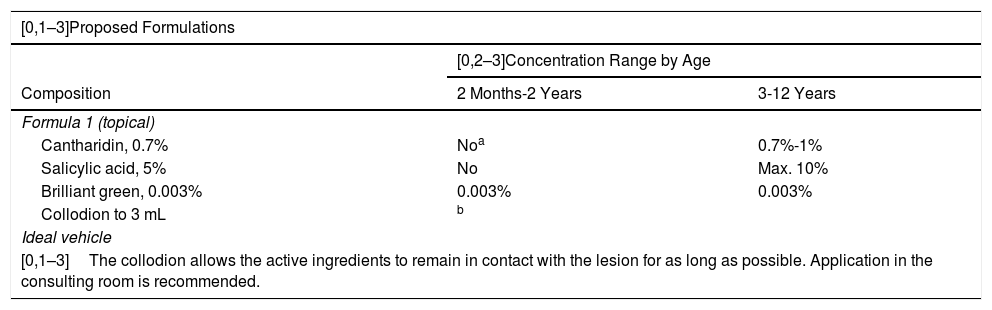
The treatment of molluscum should be tailored to the individual based on the number, size, and location of the lesions (Table 8). Occasionally, formulations with irritants and blister agents may be of use for softening the lesions in order to subsequently eliminate them by means of physical excision (curettage).
Proposed Formulas for the Treatment of Molluscum Contagiosum in Children.
| [0,1–3]Proposed Formulations | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Cantharidin, 0.7% | Noa | 0.7%-1% |
| Salicylic acid, 5% | No | Max. 10% |
| Brilliant green, 0.003% | 0.003% | 0.003% |
| Collodion to 3 mL | b | |
| Ideal vehicle | ||
| [0,1–3]The collodion allows the active ingredients to remain in contact with the lesion for as long as possible. Application in the consulting room is recommended. | ||
It has been used in children over 5 years of age, where its safety and efficacy has been demonstrated.39.
The proposed formulas are based on the use of cantharidin, a highly useful agent in this disease and one that requires particular caution due to its strength as a blister agent, to the extent that it should only be applied in the consulting room.39 Application of salicylic acid as a keratolytic agent in these formulas should be carried out with caution, as it may cause metabolic acidosis. Its use is not recommended in children under 2 years of age and its use should be limited in terms of the skin surface area to be treated and duration of treatment in older children. Although 10% KOH has been shown to be a useful alternative when applied topically, no formulations are suggested, as the product is available commercially.40
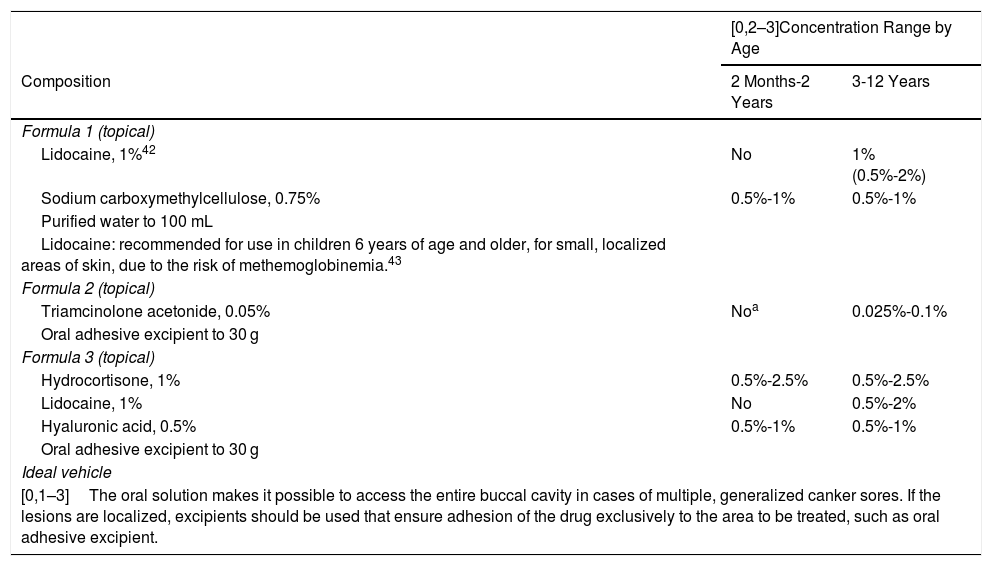
Canker SoresThe following formulations are aimed at topical treatment of the pain and inflammation associated with canker sores. Topical anesthetics such as lidocaine and corticosteroids that accelerate recovery of the mucosa and also reduce the symptoms are therefore used. Agents to protect the gastric mucosa are included in parallel, such as sucralfate or hyaluronic acid due to its demulcent properties (Table 9).41
Proposed Formulas for the Treatment of Canker Sores in Children.
| [0,2–3]Concentration Range by Age | ||
|---|---|---|
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Lidocaine, 1%42 | No | 1% (0.5%-2%) |
| Sodium carboxymethylcellulose, 0.75% | 0.5%-1% | 0.5%-1% |
| Purified water to 100 mL | ||
| Lidocaine: recommended for use in children 6 years of age and older, for small, localized areas of skin, due to the risk of methemoglobinemia.43 | ||
| Formula 2 (topical) | ||
| Triamcinolone acetonide, 0.05% | Noa | 0.025%-0.1% |
| Oral adhesive excipient to 30 g | ||
| Formula 3 (topical) | ||
| Hydrocortisone, 1% | 0.5%-2.5% | 0.5%-2.5% |
| Lidocaine, 1% | No | 0.5%-2% |
| Hyaluronic acid, 0.5% | 0.5%-1% | 0.5%-1% |
| Oral adhesive excipient to 30 g | ||
| Ideal vehicle | ||
| [0,1–3]The oral solution makes it possible to access the entire buccal cavity in cases of multiple, generalized canker sores. If the lesions are localized, excipients should be used that ensure adhesion of the drug exclusively to the area to be treated, such as oral adhesive excipient. | ||
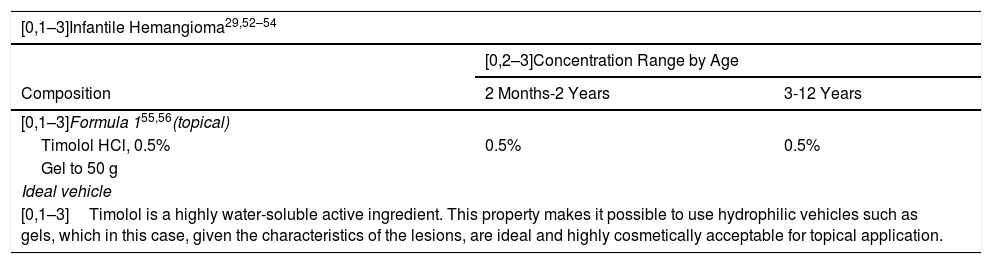
Oral propranolol is the treatment of choice in high-risk infantile hemangioma.44 The use of topical beta-blockers such as timolol, however, has been shown to be effective and safe for noncomplicated hemangiomas or even as continuation of treatment after oral propranolol is suspended.45 The contraindications and adverse effects of timolol are the same as those of propranolol, although because it is applied topically, these effects present much less frequently. It is contraindicated in patients with asthma, bradycardia, second-degree or third-degree atrioventricular block, or cardiogenic shock. Moreover, it should not be used in ulcerated hemangiomas or on mucosa. While some systemic absorption occurs, adverse effects are rare. Its use does not require monitoring. The dose is between 1 and 4 drops per day, divided into 2 applications; the drops are applied to the entire area of the hemangioma with a gentle massage. Wash hands with soap and water after application.46Table 10 shows 3 alternative formulations.
Proposed Formulas for Infantile Hemangioma, Tuberous Sclerosis, and Lamellar Ichthyosis in Children.
| [0,1–3]Infantile Hemangioma29,52–54 | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| [0,1–3]Formula 155,56(topical) | ||
| Timolol HCl, 0.5% | 0.5% | 0.5% |
| Gel to 50 g | ||
| Ideal vehicle | ||
| [0,1–3]Timolol is a highly water-soluble active ingredient. This property makes it possible to use hydrophilic vehicles such as gels, which in this case, given the characteristics of the lesions, are ideal and highly cosmetically acceptable for topical application. | ||
| [0,1–3]Tuberous Sclerosis57–59 | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| Sirolimus 0.4% | Noa | 0.4%-1%a |
| Petrolatum to 30 g (or oil-water emulsion) | ||
| [0,1–3]Ideal vehicle | ||
| [0,1–3]Because of the physical and chemical characteristics of the active ingredients, an oily vehicle, such as petrolatum, should be used, as its strong occlusive properties allow for a greater therapeutic effect. In patients with acne or acneiform reactions, an emulsion with a medium oil content may be used. | ||
| [0,1–3]Lamellar Ichthyosis | ||
|---|---|---|
| [0,2–3]Concentration Range by Age | ||
| Composition | 2 Months-2 Years | 3-12 Years |
| Formula 1 (topical) | ||
| N-acetylcysteine (NAC), 10% | 5%b | 10% |
| Urea, 5% | 5% | 5% |
| Essential oil of rosemary, 1.5% | 1.5% | 1.5% |
| Oil-water emulsion to 100 g | ||
| Formula 2 (topical) | ||
| Carbocysteine, 10% | c | 10% |
| Urea, 5% | 5% | 5% |
| Oil-water emulsion to 100 g | ||
| Ideal vehicle | ||
| [0,1–3]The vehicle should be nonionic, such as oil-water NeoPCL. The addition of an essential oil such as rosemary is useful for counteracting the intense sulfurous odor of the NAC. The addition of emollients and moisturizing agents such as glycerin, aloe vera, and oils (olive, rosehip) may also be helpful. | ||
mTOR inhibitors have been shown to be effective and safe in the treatment of tuberous sclerosis.47 Different studies have shown the efficacy of topical rapamycin in the treatment of the facial angiofibromas that are characteristic of this disease. A concentration of 1% showed the best results.48 The treatment should always be applied at night. Table 10 shows 3 a formulation of interest.
Lamellar IchthyosisIn lamellar ichthyosis, the aim is to achieve an optimum level of hydration and keratolysis of the affected areas and to modulate concentrations based on the phase of the lesion. Treatment is symptomatic, using N-acetylcysteine because of its antioxidant activity, which has proven to be effective given its ability to inhibit the proliferation of keratinocytes.49 Although irritation may appear and its characteristic sulfurous odor may complicate adherence to treatment, its efficacy is proven.50 Another formulation also exists with carbocysteine, a molecule with similar properties and with the advantage of not having an unpleasant odor, which has obtained favorable results in patients aged between 5 and 9 years.51
Table 10 shows the formulas with both active ingredients.
ConclusionsThe physiological characteristics of adults are not the same as those of children. The doses, treatment regimens, effects of potential absorption of the drug, and severity of adverse effects are not comparable, and adapting the treatments is therefore justified.
This review proposes different formulas for common skin diseases or those specific to children. Individual formulation of drugs is thus an essential therapeutic took in pediatric dermatology, providing effective, safe, quality alternatives to standard treatments.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Abarca Lachén E, Hernando Martínez P, Gilaberte Calzada Y. Revisión de las fórmulas magistrales (medicamentos individualizados) de mayor interés en dermatología pediátrica. Actas Dermosifiliogr. 2021;112:302–313.