The patient is a 58-year-old woman with a history of primary biliary cholangitis (diagnosed in 2003), gastroesophageal reflux, and depression. In 2015, she was evaluated by the rheumatology department for pain in the wrist, hip, and knee joints that had appeared 2 years earlier. On questioning, she reported fatigue and Raynaud phenomenon but did not report photosensitivity or alopecia. The physical examination was normal. A polyarthralgia study was initiated and treatment with 200mg/d hydroxychloroquine was indicated, after which, symptoms ceased (Raynaud phenomenon included). Tests included capillaroscopy, which revealed periungual telangiectasias. Other tests were normal (blood count, HSV, thyroid profile, ANA, ENA, anti-DNA, VDRL, C3-C4 complement, immunoglobulins). The clinical picture was interpreted as mixed connective tissue disease.
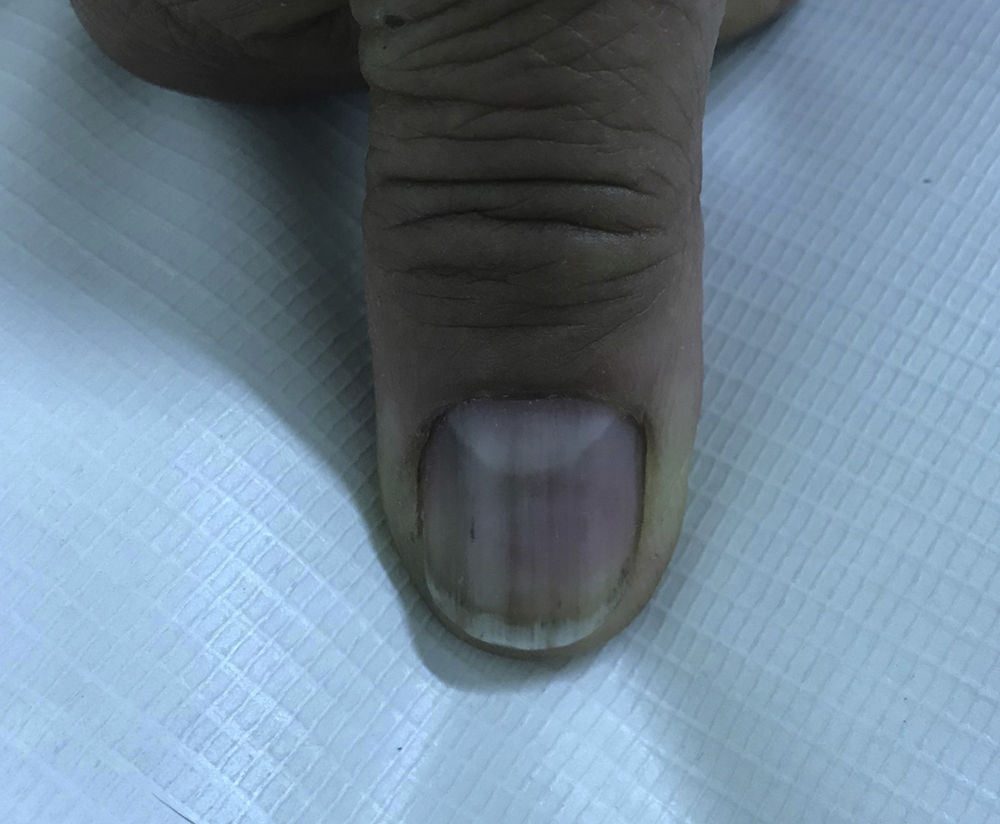
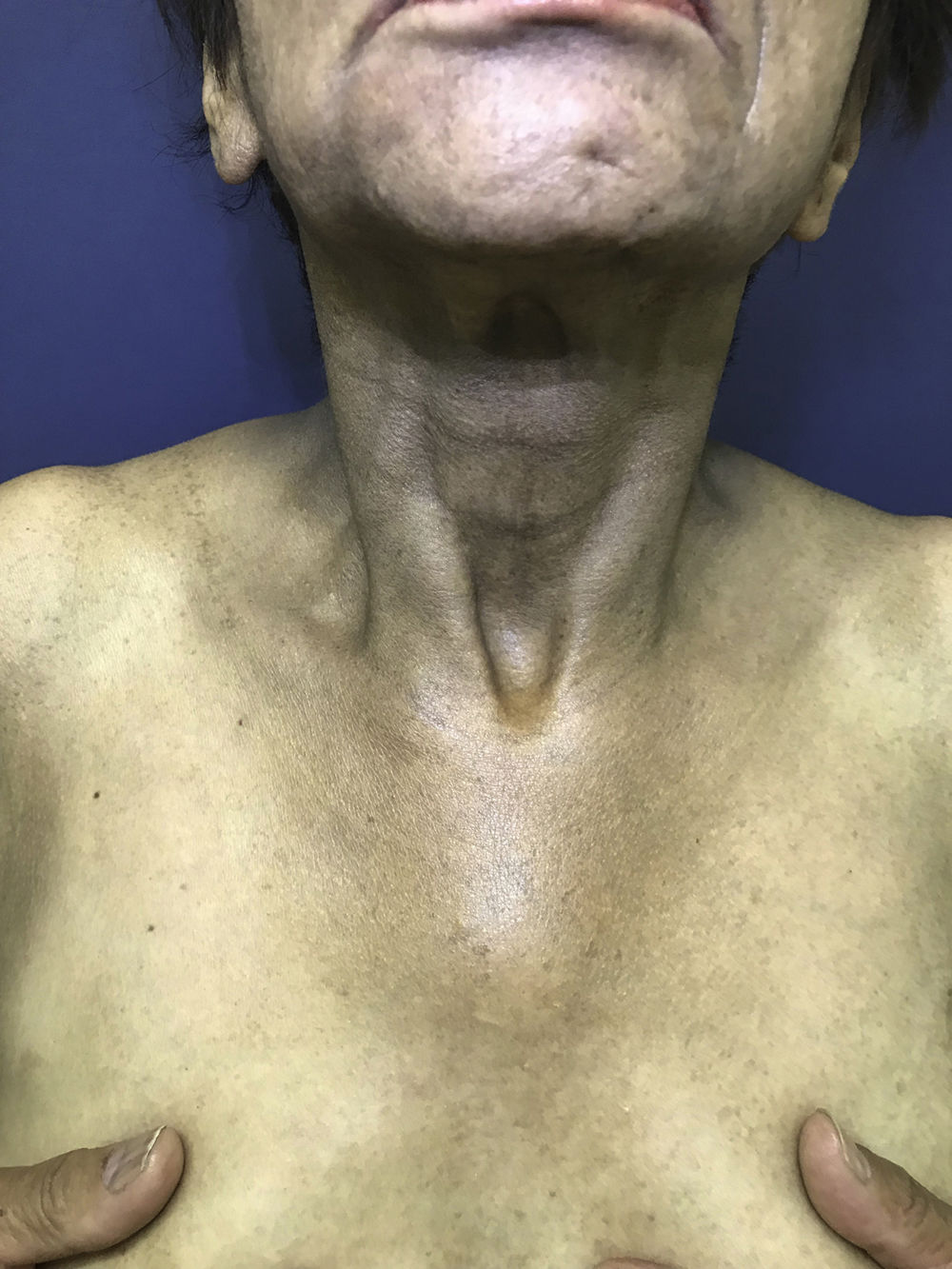
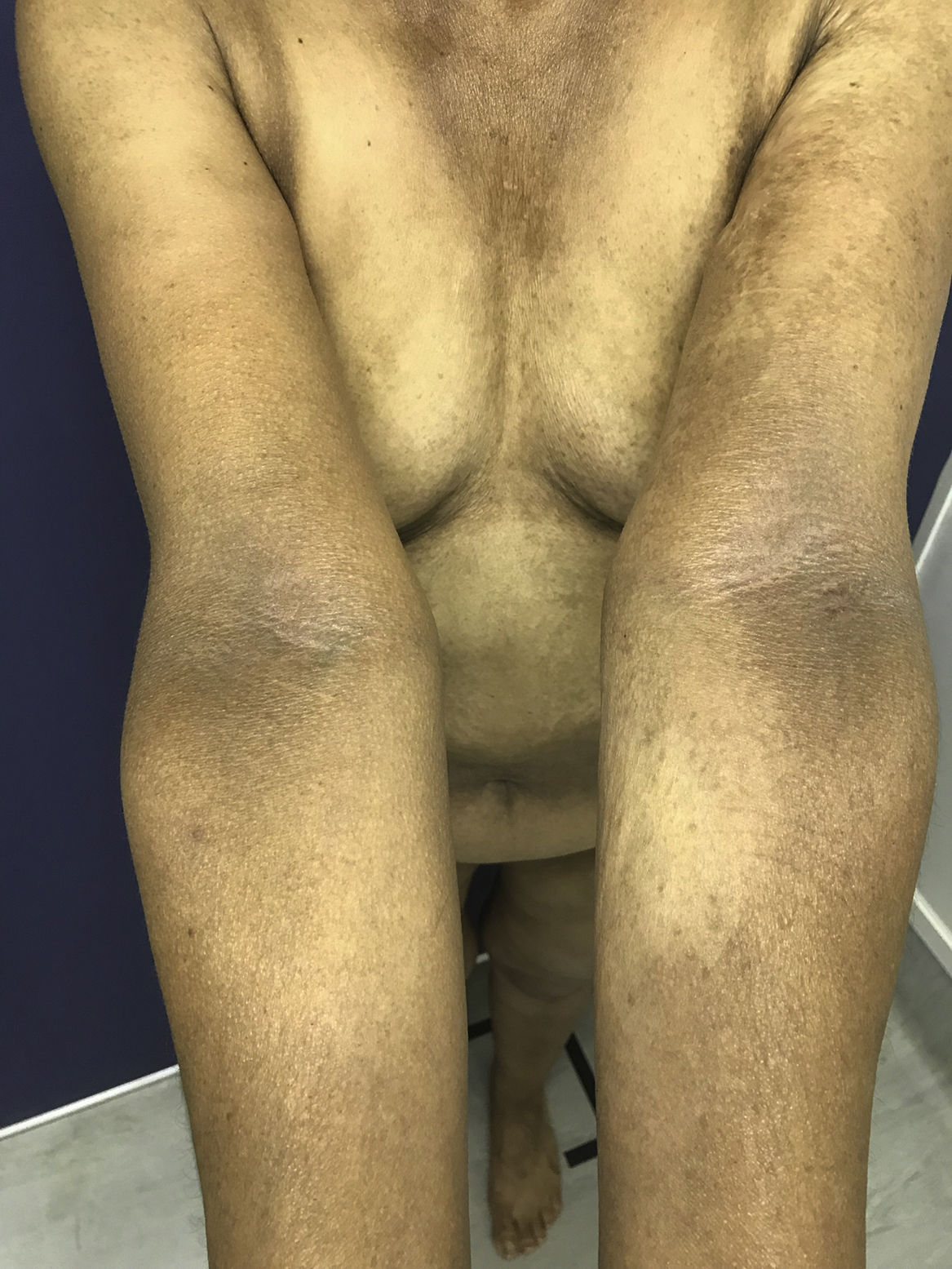
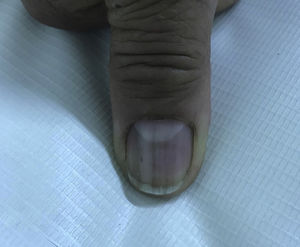
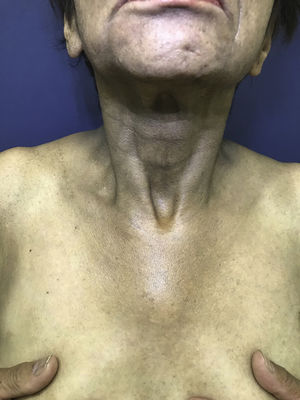
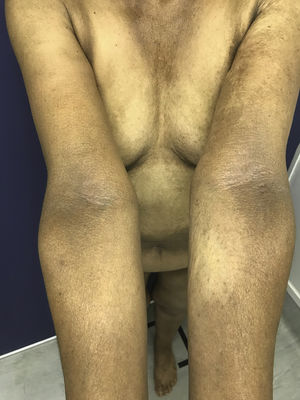
In 2018, the patient was referred to the dermatology department due to symptoms that had appeared 2 years earlier, characterized by generalized, progressive darkening of the skin, mainly involving the neck and arms. The patient uses hydroxychloroquine, folic acid, calcium, ursodeoxycholic acid, omeprazole, venlafaxine, and zopiclone. The patient has no allergies. Fitzpatrick skin type III. The physical examination revealed hyperpigmented dark coffee-coloured patches (neck, back, and upper limbs) associated with longitudinal melanonychia in all the nails (hands and feet); the oral mucosa was not involved. Skin biopsies (scapula and left ulna) were compatible with postinflammatory hyperpigmentation. Questioning of the patient revealed that the timing of the symptoms coincides with the start of hydroxychloroquine administration (Figs. 1–3).
What is Your Diagnosis?
Diagnosis and CommentsSkin hyperpigmentation secondary to the use of antimalarial drugs is a common adverse effect (10%-25%), mainly caused by chloroquine.1–3 The first case was reported in 1944 during World War II, associated with the use of mepacrine as prophylaxis against malaria.4 Hydroxychloroquine is currently the most widely used drug within this group. It is frequently prescribed in rheumatologic diseases due to its immunosuppressant and anti-inflammatory properties. It also presents a lower rate of adverse effects, the most important of which is involvement of the eye.1,3
Few cases of skin hyperpigmentation induced by hydroxychloroquine have been reported in the literature and nail involvement is the rarest adverse effect.2 It manifests as patches of variable color (grayish-blue, coffee-yellow, or dark purple), which can appear on the oral mucosa (hard palate and gums) and skin, mainly on the face, torso, back, nails, anterior surface of the arms, hands, legs, and feet.2,5 The lesions appear between 4 and 70 months after start of treatment.2 In terms of pathophysiology, hydroxychloroquine has been shown to deposit and become fixed in melanin-containing tissue, although the exact mechanism of hyperpigmentation is unknown.3 Longitudinal melanonychia appears when the melanocytes of the nail matrix are stimulated for some reason (trauma, systemic disease, iatrogenic effects, etc.), which causes increased activity or hyperplasia resulting in pathological depositing of melanin in the nail plate.6
Some studies have linked the appearance of melanonychia with ecchymosis, concomitant use of antiplatelet therapy and oral anticoagulant treatment. For this reason, it has been suggested that a high hemosiderin content may increase the activity of the melanocytes in this context.1,5
The differential diagnosis should rule out metabolic causes (hemochromatosis, late-onset cutaneous porphyria, pellagra), endocrinologic causes (hyperthyroidism, Addison disease, Nelson syndrome, ectopic ACTH secretion), autoimmune causes (systemic sclerosis, primary biliary cholangitis), neoplastic causes (lung cancer, metastatic melanoma, mycosis fungoides), metal poisoning (gold, silver), and drugs (NSAIDs, chlorpromazine, amiodarone, antitumor drugs, and tetracycline).2
After suspending the hydroxychloroquine, the lesions have gradually disappeared; this is an expected response in signs and symptoms caused by the adverse effects of a drug. No involvement of the eyes was observed. Although primary biliary cirrhosis is a cause of hyperpigmentation, it was ruled out by the time since onset (15 years) and the response of the skin to the start and suspension of the drug. The clinical signs and symptoms and the studies carried out in this patient ruled out other etiologies. It is possible that the hyperpigmentation may not resolve completely. This first reported case in Chile allows us to describe a potentially reversible manifestation that, if not identified, may have a considerable aesthetic and psychological impact on patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cristián Fischer L, Matías Sanhueza M. Hiperpigmentación cutánea y melanoniquia provocada por hidroxicloroquina: primer caso reportado en Chile. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2019.02.027