Psoriasis is a chronic inflammatory skin disease that can be treated with several currently available drugs. Until November 2018, the 2 anti-interleukin (IL) 17 drugs available in Spain were secukinumab and ixekizumab. There is little evidence on whether the lack of response to either of these drugs can also predict the response to other drugs with the same mechanism. The literature shows that affected patients could benefit from treatment with the other available drug,1–4 although there are no clear guidelines on which is the best treatment after failure of an initial biologic drug.5 The objective of our study was to determine whether the inefficacy or loss of efficacy of one of the anti-IL-17 drugs points to the failure of another one.
We designed a single-center, retrospective, longitudinal, observational study that included patients with moderate to severe plaque psoriasis. Patients were aged >18 years and had been treated with the 2 anti-IL-17 drugs available in Spain up to November 2018, namely, secukinumab and ixekizumab. Both drugs were administered according to the summary of product characteristics. All of the patients had reported that one of the drugs had proven ineffective and that they had subsequently received treatment with the other drug for at least 12 weeks. The patients’ demographic characteristics were reviewed. For each treatment, we analyzed the initial Psoriasis Area Severity Index (PASI), the minimum PASI reached, and time until it was reached. The effectiveness of both drugs was evaluated using the absolute PASI. A PASI ≤ 3 was considered an optimal response, and a PASI 3-5 at any time from initiation of treatment was considered an acceptable response. Primary inefficacy was defined as not reaching at least a 50% improvement in the PASI at any of the medical visits; secondary inefficacy (or loss of efficacy) was defined as not maintaining a PASI 50 once it was reached.
We also recorded the PASI when it was decided to switch to the second drug, the reason for switching the first drug, concomitant systemic treatment, and adverse effects.
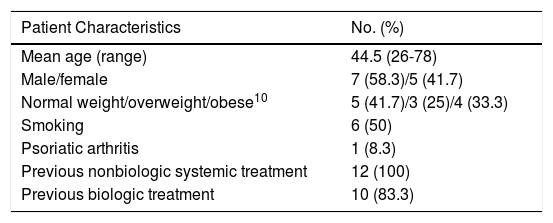
The characteristics of the sample are shown in Table 1. Ten patients received secukinumab as their first drug, followed by ixekizumab. The order was reversed in the other 2 patients.
Epidemiologic Characteristics of the Study Sample.
| Patient Characteristics | No. (%) |
|---|---|
| Mean age (range) | 44.5 (26-78) |
| Male/female | 7 (58.3)/5 (41.7) |
| Normal weight/overweight/obese10 | 5 (41.7)/3 (25)/4 (33.3) |
| Smoking | 6 (50) |
| Psoriatic arthritis | 1 (8.3) |
| Previous nonbiologic systemic treatment | 12 (100) |
| Previous biologic treatment | 10 (83.3) |
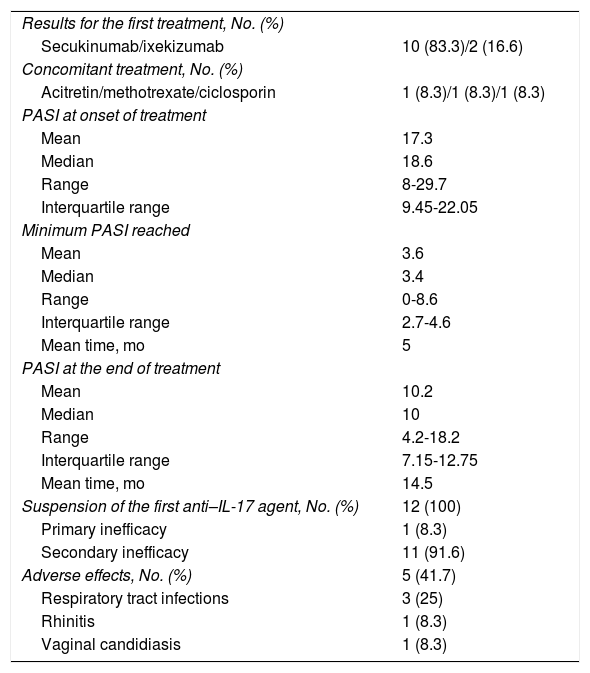
With respect to the first treatment (Table 2), the mean PASI at initiation of the first drug was 17.3. The mean minimum PASI was 3.6 (5 months of treatment). An optimal response was observed in only 6 patients. The mean PASI when it was decided to switch treatment was 10.2 after 14.5 months. Mild adverse effects were recorded in 5 patients.
Results for the First Anti–IL-17 Agent.
| Results for the first treatment, No. (%) | |
| Secukinumab/ixekizumab | 10 (83.3)/2 (16.6) |
| Concomitant treatment, No. (%) | |
| Acitretin/methotrexate/ciclosporin | 1 (8.3)/1 (8.3)/1 (8.3) |
| PASI at onset of treatment | |
| Mean | 17.3 |
| Median | 18.6 |
| Range | 8-29.7 |
| Interquartile range | 9.45-22.05 |
| Minimum PASI reached | |
| Mean | 3.6 |
| Median | 3.4 |
| Range | 0-8.6 |
| Interquartile range | 2.7-4.6 |
| Mean time, mo | 5 |
| PASI at the end of treatment | |
| Mean | 10.2 |
| Median | 10 |
| Range | 4.2-18.2 |
| Interquartile range | 7.15-12.75 |
| Mean time, mo | 14.5 |
| Suspension of the first anti–IL-17 agent, No. (%) | 12 (100) |
| Primary inefficacy | 1 (8.3) |
| Secondary inefficacy | 11 (91.6) |
| Adverse effects, No. (%) | 5 (41.7) |
| Respiratory tract infections | 3 (25) |
| Rhinitis | 1 (8.3) |
| Vaginal candidiasis | 1 (8.3) |
The first treatment was suspended in all patients owing to primary inefficacy of ixekizumab in the case of 1 patient and secondary inefficacy in the remaining 11 patients.
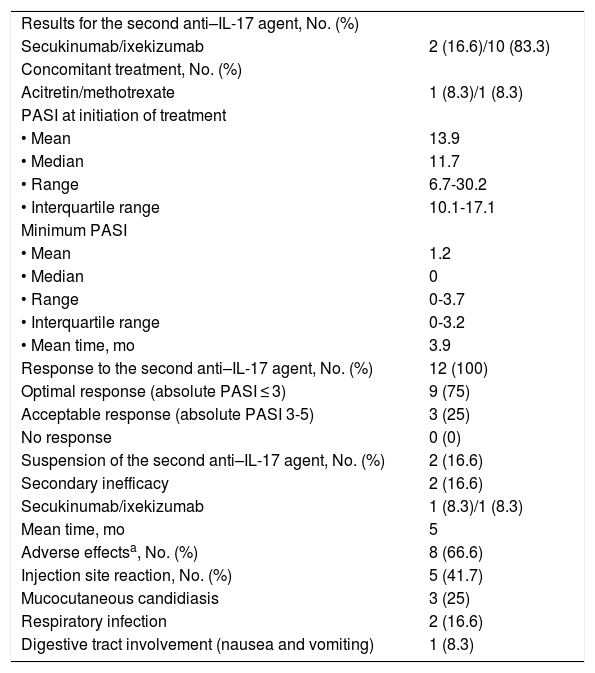
The results for the second treatment with an anti-IL-17 agent are shown in Table 3. In this case, the mean PASI at initiation of the second drug was 14. The minimum PASI reached was 1.2 after 3.9 months of treatment.
Results for the Second Anti–IL-17 Agent.
| Results for the second anti–IL-17 agent, No. (%) | |
| Secukinumab/ixekizumab | 2 (16.6)/10 (83.3) |
| Concomitant treatment, No. (%) | |
| Acitretin/methotrexate | 1 (8.3)/1 (8.3) |
| PASI at initiation of treatment | |
| • Mean | 13.9 |
| • Median | 11.7 |
| • Range | 6.7-30.2 |
| • Interquartile range | 10.1-17.1 |
| Minimum PASI | |
| • Mean | 1.2 |
| • Median | 0 |
| • Range | 0-3.7 |
| • Interquartile range | 0-3.2 |
| • Mean time, mo | 3.9 |
| Response to the second anti–IL-17 agent, No. (%) | 12 (100) |
| Optimal response (absolute PASI ≤ 3) | 9 (75) |
| Acceptable response (absolute PASI 3-5) | 3 (25) |
| No response | 0 (0) |
| Suspension of the second anti–IL-17 agent, No. (%) | 2 (16.6) |
| Secondary inefficacy | 2 (16.6) |
| Secukinumab/ixekizumab | 1 (8.3)/1 (8.3) |
| Mean time, mo | 5 |
| Adverse effectsa, No. (%) | 8 (66.6) |
| Injection site reaction, No. (%) | 5 (41.7) |
| Mucocutaneous candidiasis | 3 (25) |
| Respiratory infection | 2 (16.6) |
| Digestive tract involvement (nausea and vomiting) | 1 (8.3) |
Abbreviation: IL, interleukin; PASI, Psoriasis Area Severity Index.
All patients in our sample responded to treatment with a second anti-IL-17 agent after failure of the first. An optimal response was recorded in 75% and an acceptable response in the remaining 25% (3 patients). Adverse effects—all mild—were observed in 8 patients.
In the present study, the sequence of treatment in 10 of the 12 patients was secukinumab first followed by ixekizumab. The mean PASI at initiation of the first drug was greater, 17 compared with 14 for the second drug, although the ranges were similar in both groups. A more favorable response in less time was observed with the second treatment.
In the present series, all patients whose first anti-IL-17 agent had failed responded to treatment with the other anti-IL-17 drug. These results are similar to those published elsewhere.2–4 The only series that evaluated absolute PASI was that of Conti et al.,3 where 79.7% of patients reached PASI ≤ 3 at week 12 of treatment. In these studies, secukinumab failed first in all cases. The reason for these findings is unknown, although there may be an association with the greater affinity of ixekizumab for IL-17A.6,7
The frequency of adverse reactions was greater with the second treatment than with the first. In the case of the second drug, injection site reaction was noteworthy. This was observed in 5 patients, all of whom were taking ixekizumab, and was more common than reported in clinical trials, i.e., between 6.4% and 21.4%.8,9
In our experience, all of those patients who did not experience a primary or secondary response to an anti-IL-17 drug benefited from switching to another drug aimed at the same therapeutic target.
Conflicts of interestBelén Pinilla Martín, Alba Sánchez Velázquez, and Cristina Vico Alonso have acted as researchers for Almirall, AbbVie, and UCB.
Raquel Rivera Díaz has provided consultancy services to AbbVie, Almirall, Janssen, Lilly, and Pfizer. She has acted as a speaker for MSD, AbbVie, Janssen, LEO Pharma, Novartis, and Pfizer and as a researcher for AbbVie, Pfizer, Janssen, Celgene, Lilly, Novartis, and LEO Pharma.
Please cite this article as: Pinilla Martín B, Sánchez Velázquez A, Vico Alonso C, Rivera Díaz R. De un anti-IL-17 a otro: dar una segunda oportunidad o cambiar de diana. Actas Dermosifiliogr. 2021;112:473–475.