Current trends in our setting indicate that the prevalence of actinic keratosis and similar diseases will increase in coming years and impose a greater burden on health care resources. A long list of clinical features must be taken into account when approaching the treatment of actinic keratosis. Until recently, therapeutic approaches focused solely on ablative procedures and the treatment of individual lesions and did not take into account areas of field cancerization. Now that the therapeutic arsenal has grown, standardized criteria are needed to guide the optimal choice of treatment for each patient. The elaboration of evidence-based consensus recommendations for the diagnosis and treatment of actinic keratosis generates knowledge that will help clinicians to deliver the highest level of care possible, standardizing decision-making processes and enhancing awareness among all the health professionals involved in the care pathway.
Las características de nuestro entorno sugieren que enfermedades como la queratosis actínica (QA) aumentarán su prevalencia y, en consecuencia, la demanda asistencial en los próximos años. Deben tenerse en cuenta una extensa lista de características clínicas en el abordaje terapéutico de la QA, hasta hace poco compuesto únicamente por técnicas ablativas y exclusivamente dirigidas a las lesiones, sin considerar el campo de cancerización. El incremento del arsenal terapéutico de los últimos años hace necesaria la homogenización de criterios que faciliten la elección de la mejor opción para cada paciente. La formulación de recomendaciones de consenso entre expertos a partir de la revisión de las evidencias científicas en cuanto a diagnóstico y tratamiento disponibles, permite aportar conocimiento dirigido a la mayor calidad en la atención de los pacientes, facilita una mayor homogeneidad en la toma de decisiones y promueve la sensibilización necesaria de todos los agentes sanitarios involucrados.
The prevalence of actinic keratosis (AK) is difficult to estimate because case registers have not been established and few studies have focused specifically on prevalence. Available epidemiologic data indicate a high prevalence of the condition in populations with certain skin phototypes (I to III) as well as a rise in the number of cases in recent decades.1–6 In the United Kingdom, a prevalence of 15% in men and 6% in women has been documented.7 Estimates vary considerably from one country to another and according to the age of the population studied. The UK study found rates over 34% in age groups over 70 years. A European guideline on this disease8 concluded that AK is increasing in prevalence; it affects millions of patients worldwide and is becoming the most common in situ carcinoma in humans.
The European guideline8 begins by summarizing available evidence on the etiology and pathogenesis of AK as well as the histopathology and clinical manifestations of the disease; it also reviews the wide range of treatment options available. This document should be used to orient decision-making even though specific recommendations were not formulated in it. The aim of the present article is to facilitate greater consistency in making decisions about the management of symptoms and treatment of AK. The emphasis is on the importance of early diagnosis and treatment of lesions to prevent their progression to invasive squamous cell carcinoma (SCC).
MethodsThe basis for this consensus paper is the 2011 European guideline8 and its adaptation to the Spanish context through structured, participatory consensus building among experts, who are the drafters of the recommendations and authors of the paper.
The working group consisted of 7 specialists and 1 primary care physician who attend patients in different Spanish health care facilities. The group first made a technical translation of the European guideline8 in order to subject it to critical reading focused on the objectives the group prioritized. The information in the guideline was then synthesized to establish recommendations applicable to Spain. The experts also conducted a further review of the literature published after the guideline appeared. They then incorporated evidence from the new literature and drafted preliminary recommendations for the evaluation and treatment of AK, also providing additional general and conceptual reflections on the disease.
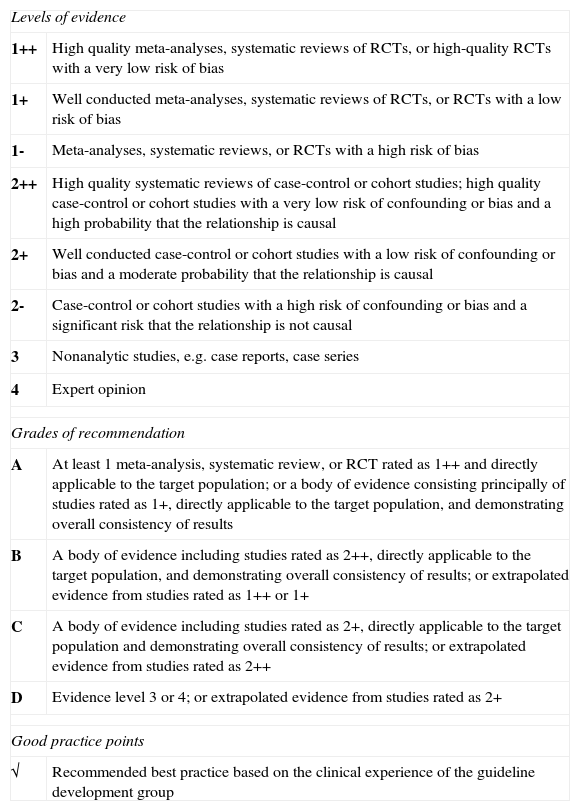
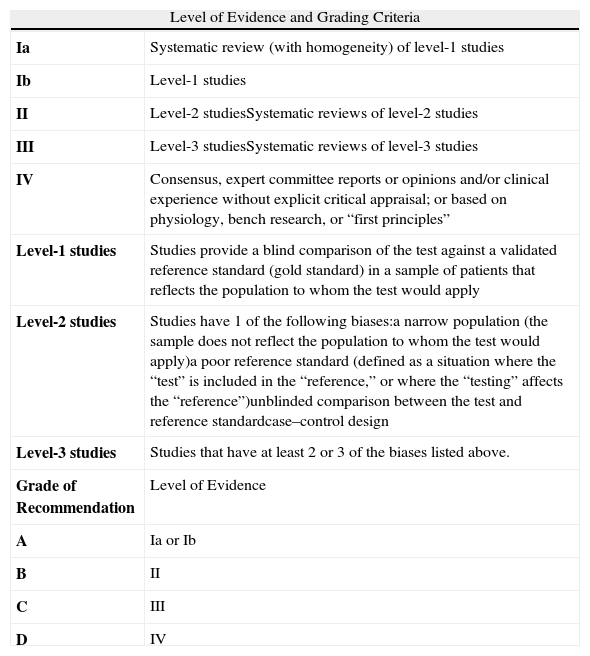
The first draft of this paper was debated and revised in a structured meeting in which all members of the expert working group participated. Once the experts approved the draft, each recommendation was graded and classified according to level of evidence using the criteria of the Scottish Intercollegiate Guidelines Network9 (Table 1) and the National Institute for Clinical Excellence10 in the case of evidence for diagnostic tests (Table 2). The final manuscript was approved by all participants.
Levels of Evidence and Grading of Recommendations According to the Scottish Intercollegiate Guidelines Networka
| Levels of evidence | |
| 1++ | High quality meta-analyses, systematic reviews of RCTs, or high-quality RCTs with a very low risk of bias |
| 1+ | Well conducted meta-analyses, systematic reviews of RCTs, or RCTs with a low risk of bias |
| 1- | Meta-analyses, systematic reviews, or RCTs with a high risk of bias |
| 2++ | High quality systematic reviews of case-control or cohort studies; high quality case-control or cohort studies with a very low risk of confounding or bias and a high probability that the relationship is causal |
| 2+ | Well conducted case-control or cohort studies with a low risk of confounding or bias and a moderate probability that the relationship is causal |
| 2- | Case-control or cohort studies with a high risk of confounding or bias and a significant risk that the relationship is not causal |
| 3 | Nonanalytic studies, e.g. case reports, case series |
| 4 | Expert opinion |
| Grades of recommendation | |
| A | At least 1 meta-analysis, systematic review, or RCT rated as 1++ and directly applicable to the target population; or a body of evidence consisting principally of studies rated as 1+, directly applicable to the target population, and demonstrating overall consistency of results |
| B | A body of evidence including studies rated as 2++, directly applicable to the target population, and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 1++ or 1+ |
| C | A body of evidence including studies rated as 2+, directly applicable to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2++ |
| D | Evidence level 3 or 4; or extrapolated evidence from studies rated as 2+ |
| Good practice points | |
| √ | Recommended best practice based on the clinical experience of the guideline development group |
Abbreviation: RCT, randomized clinical trial.
Levels of Evidence for the Accuracy of Diagnostic Tests and Grading of Recommendationsa
| Level of Evidence and Grading Criteria | |
| Ia | Systematic review (with homogeneity) of level-1 studies |
| Ib | Level-1 studies |
| II | Level-2 studiesSystematic reviews of level-2 studies |
| III | Level-3 studiesSystematic reviews of level-3 studies |
| IV | Consensus, expert committee reports or opinions and/or clinical experience without explicit critical appraisal; or based on physiology, bench research, or “first principles” |
| Level-1 studies | Studies provide a blind comparison of the test against a validated reference standard (gold standard) in a sample of patients that reflects the population to whom the test would apply |
| Level-2 studies | Studies have 1 of the following biases:a narrow population (the sample does not reflect the population to whom the test would apply)a poor reference standard (defined as a situation where the “test” is included in the “reference,” or where the “testing” affects the “reference”)unblinded comparison between the test and reference standardcase–control design |
| Level-3 studies | Studies that have at least 2 or 3 of the biases listed above. |
| Grade of Recommendation | Level of Evidence |
| A | Ia or Ib |
| B | II |
| C | III |
| D | IV |
By consensus, the experts agreed that AK lesions are in situ SCCs at low risk of progressing to invasive disease and that they manifest as slightly erythematous lesions that are scaly or rough to the touch; they are found on chronically sun-damaged skin. Because the evidence to support this definition is currently a subject of debate, the authors note that the definition agreed upon is the result of expert opinion (evidence level 4, grade D recommendation).
Etiology and PathogenesisAs stated in the European guideline,8 “AKs are mainly caused by non-ionising radiation, especially through ultraviolet light associated with chronic sun exposure. While UV-A (320– 400nm) induced photo-oxidative stress indirectly induces characteristic DNA mutations, the spectrum of UV-B (290–320nm) irradiation directly results in the formation of cyclobutane (thymin) dimer formation in DNA and RNA. In the absence of appropriate repair mechanisms, these DNA changes represent the initiation of keratinocyte mutations which can progress into the development of AKs.” The guideline then cites Brash et al.11
The guideline8 continues: “Other factors like repeated iatrogenic exposure to UV-A, with or without combination with psoralenes, x-rays or radioisotopes are known to induce AKs,” further noting the co-carcinogenic role of human papilloma viruses in the etiopathogenesis of AKs (citing Lober and Lober12 and Stockfleth et al.13). Implicated would be the interaction of the E6 and E7 HPV oncoproteins with the proapoptotic Bak protein.14–18
Risk FactorsCumulative UV light exposure is the main risk factor, but associated factors include advanced age, male sex, outdoor occupations (e.g., farming or seagoing occupations) and recreational activities (e.g., tennis, golf), place of residence (high altitude, latitudes closer to the equator), and exposure to artificial UV radiation. An individual's sensitivity to UV light determined by skin phototype (I and II), chronic iatrogenic immunodeficiency (e.g., in organ transplant patients), genetic syndromes that undermine DNA repair mechanisms or chromosome stability, photosensitivity and exposure to certain toxins or drugs that affect the cell cycle (e.g., hydroxyurea or arsenic, and various biologic agents used in oncology are also probably implicated).19–21
Field CancerizationThe term field cancerization was introduced to refer to the development of multiple primary tumors in an area that has been genetically altered by a common carcinogen.22 In the case of AK, the field would be an area of sun-damaged skin that can be found surrounding each AK lesion and that displays the same genetic changes found in the lesion itself. The field can contain clinically visible AKs, subclinical AKs (only visible under a microscope), and groups of keratinocytes with genetic mutations detectable only with molecular biology methods.22 The phenomenon of field cancerization has important therapeutic implications that will be discussed below.
Clinical and Pathologic Classifications of AKAKs are characterized microscopically by proliferation of intraepidermal keratinocytic atypia (large pleomorphic and hyperchromatic nuclei) with loss of polarity and mitotic figures; these cells are similar to the keratinocytes in invasive SCCs and for this reason AKs are considered carcinomas in situ23–27 (level of evidence 4, grade D recommendation). These aberrations represent the first stage, characterized by keratinocytic atypia,1 of a clinical course that may progress to invasive SCC. In addition to pathologic evidence of this progression, there are indirect indications of the relationships between the in situ and invasive lesions; for example 80% of invasive SCCs show AKs in margins and other evidence can be found at the molecular level.28 Three histologic grades of AK can be distinguished on the basis of degree of intraepidermal involvement of keratinocytic atypia29: AK-I, in the lower third of the epidermis; AK-II, in the lower two-thirds of the epidermis; and AK-III, affecting the full thickness of the epidermis. Clinical phenotypes of AK have been defined on the basis of the intensity or predominance of characteristic signs (Table 3).
Clinical Variants of AK.
| Hypertrophic or hyperkeratotic AK |
| Highly keratotic papule or plaque on an inflammatory substrate. The lesion is highly visible. When the keratotic component is exuberant, a cutaneous horn develops. |
| Pigmented AK |
| A macule, or flat papule, that is hyperkeratotic, hyperpigmented, or reticulated but lacks an erythematous component. This type must be distinguished from lentigo maligna, pigmented basal cell carcinoma, and reticulated seborrheic keratosis. Dermoscopy is useful for differential diagnosis, although biopsy may be necessary. |
| Lichenoid AK |
| Clinically similar to the classic form of AK, but more pronounced erythema around the base of the lesion makes there appear to be an underlying lichenoid infiltrate. |
| Atrophic AK |
| Erythematous macule that is somewhat scaly. Histology shows an atrophic epidermis. |
Abbreviation: AK, actinic keratosis.
Diagnosis of AK is mainly clinical. However, histopathologic confirmation may be required to distinguish AKs from other lesions and is especially necessary when there may be dermal invasion and, therefore, transformation to invasive SCC. Although there are no clear clinical signs to indicate progression to invasive disease, this possibility should be suspected, at least initially, when a lesion appears inflamed, indurated, ulcerated or large (> 2 cm). Other signs of possible progression are bleeding, rapid growth, lack of response to appropriate treatment, or recurrence after successful treatment. To distinguish AK from other possible lesions when the clinical diagnosis is doubtful, and whenever there is suspicion of transformation to invasive SCC, the lesion should be biopsied (level of evidence 4, grade D recommendation).
Several diagnostic tools have proven useful for differential diagnosis. Dermoscopy can help distinguish AK from superficial basal cell carcinoma, or differentiate between pigmented AK and lentigo maligna or pigmented basal cell carcinoma. Confocal scanning laser microscopy has been reported to have good diagnostic sensitivity and specificity,30 but at least in Spain, this tool is available in few hospitals and so is currently used more for research than routine clinical care. Photodynamic therapy (PDT) adapted for diagnosis provides another method. While the efficacy of this resource has not been fully confirmed, it seems it can help identify the limits of field cancerization.31 However, PDT is not available in all centers and so trying this approach would require investment.
Clinical Course and PrognosisThe natural history of AKs may proceed in any of 3 ways. They may regress spontaneously, remain AKs, or progress to invasive SCC. As the course they will take cannot be predicted, treatment is advisable. In 1977, Harvey et al.3 reported a rate of spontaneous regression between 15% and 55% for AK within the year. The percentage of AKs that become invasive SCCs has been the focus of several studies with varying designs and disparate results. However, it must be remembered that over 80% of invasive SCCs on exposed areas develop on or adjacent to an AK lesion.28,32–34 In a prospective study that followed patients for 12 months, Marks et al.35 reported an incidence of progression to invasive SCC within the year of 0.24%. Most patients have more than a single lesion, however, so the risk of progression to invasive SCC over 10 years would be between 6.1% and 10.2%36; that figure rises to 40% in immunodeficient patients,37–40 as has been noted by the European guideline.8 It is important to remember that AKs are considered a sign of chronic actinic damage and identify a group of patients at high risk for nonmelanoma cancer41 (level of evidence 4, grade D√ recommendation).
Prevention and Follow-UpBoth primary and secondary measures are available to prevent AK. Information and education on sun-protection measures are fundamental for primary prevention. Such steps are especially important for transplant recipients, who are at very high risk for AK and invasive SCC and must start to take precautions as soon as they are placed on a wait list.8,42,43 Secondary measures include self-examination even before consulting a physician because early detection is of great importance for achieving a cure and preventing progression to invasive SCC. Products that combine sun screens with DNA reparative agents are currently being tested.44
In transplant patients, prevention must be stressed and careful inspection at frequent follow-up visits (every 3 or 4 months) will be essential. Several studies in the general population and in transplant recipients have demonstrated that sun-protection behaviors are effective not only for preventing new lesions but also for clearing existing ones.45–48
Recurrence is common in these patients and the appearance of new lesions in the cancerized field is a marker of chronic sun damage and thus a risk factor for the development of invasive disease; therefore, the patient with AKs should be followed at individualized intervals based on the number of lesions, patient characteristics, associated risk factors, and other relevant aspects, with insistence on early diagnosis and treatment and the importance of sun protection2749–52 (level of evidence 4, grade D recommendation).
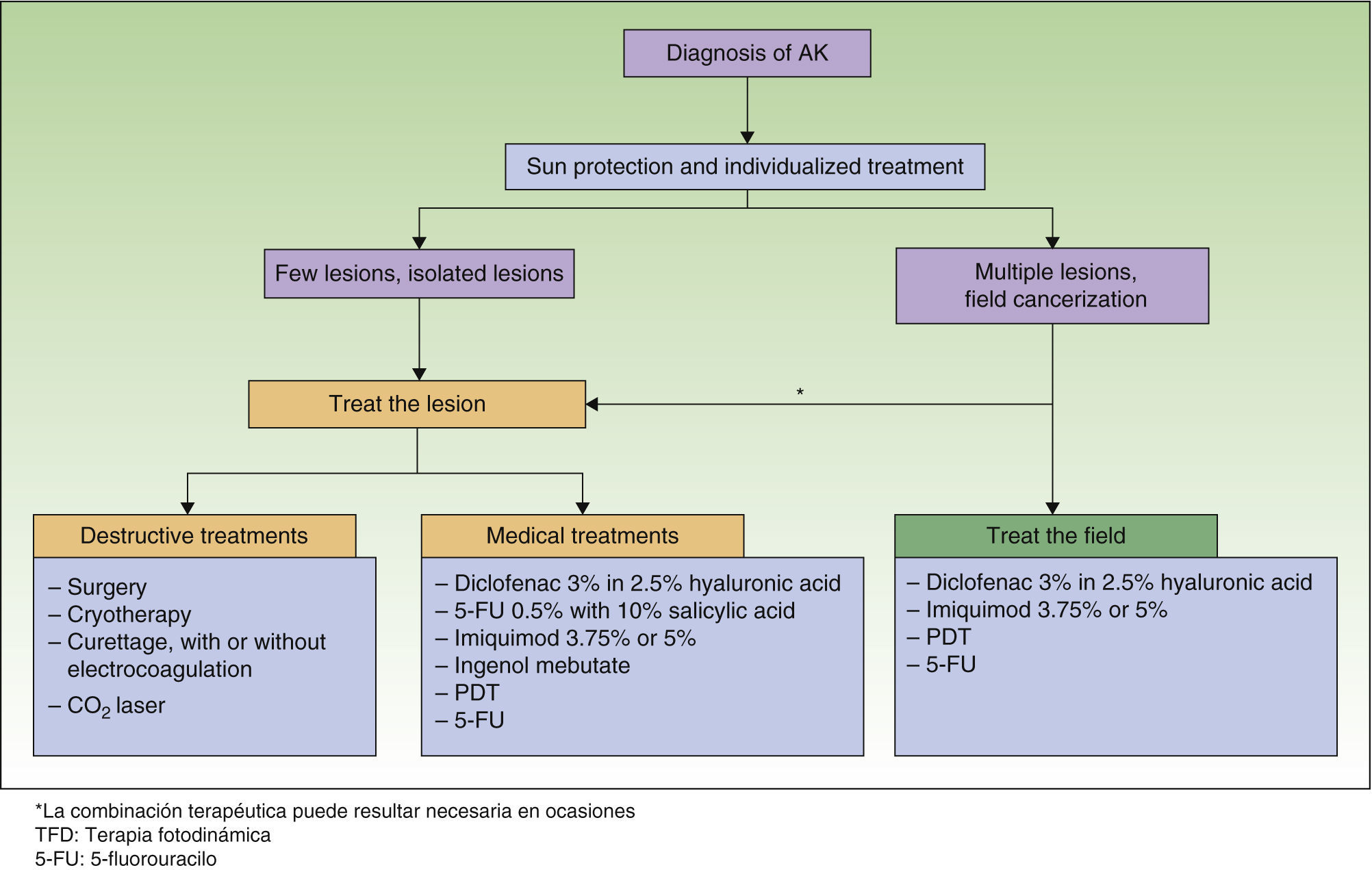
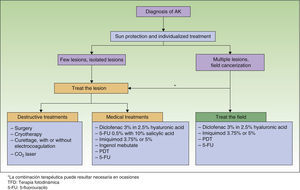
Treatment Algorithm Based on Expert ConsensusThe authors of the present paper seem to have reached agreement on recommending that all AKs be treated because they are considered carcinomas in situ that may progress to invasive SCC6,8,22 (level of evidence 4, grade D recommendation). The treatment algorithm developed by Stockfleth et al.27 states that after AK is diagnosed, sun protection and individually tailored treatment should be initiated. When lesions are few and isolated, treatment should target individual lesions. When lesions are many or field cancerization is suspected, treatment should target both the field and the specific lesion (Fig. 1).
The presence of field cancerization has important implications for treatment. If a lesion is the sole target for treatment, or only clinically visible lesions are targeted, they will be eliminated but the field will continue to be a problem, giving rise to new AKs over time.49 If the field is treated, however, visible and subclinical AKs will also be covered, along with clones of cells that will become AKs. This approach thus prevents both new AKs and invasive SCC; periods of remission will be longer, and AK treatment sessions can be scheduled farther apart.27 Whenever possible, therefore, field cancerization should be targeted for treatment with the aim of preventing the progression of subclinical lesions to visible ones, which in turn might progress further to become invasive SCCs (level of evidence 4, grade D√ recommendation).
The physician must weigh the various factors relevant to each case and choose a tailored treatment approach. It is important to remember that combining destructive treatments (generally, those that target the lesion) and topical ones (useful for treating both an AK and field cancerization) may be advisable in some circumstances, such as when there is progression to invasive SCC (level of evidence 4, grade D√ recommendation). Treatment combinations may be required to first eliminate the lesion by destroying it and then treating the field. The choice of a destructive treatment combined with medical treatment will depend on patient profile, lesion characteristics, what options are locally available, and other constraints at the time of treatment. Topical treatment with PDT and imiquimod may also be a beneficial combination. The clinical and histologic response to these treatments in combination is better than the response to monotherapy with either alone; tolerance is also better.50 Little is currently known about the usefulness of topical treatment combinations, although the possibility of unknown interactions should be taken into consideration.
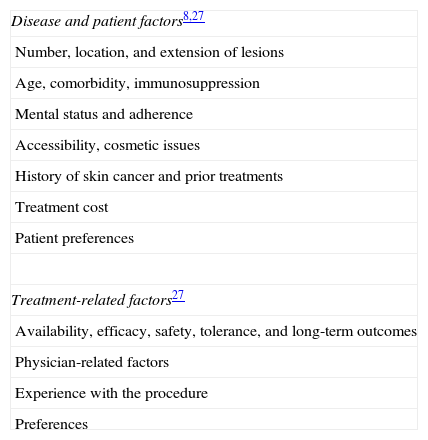
Therapeutic OptionsThe decision to target the lesion or to treat field cancerization, as well as the choice of modality, will be affected by a number of factors (Table 4).
Factors That Affect Choice of Treatment.
| Disease and patient factors8,27 |
| Number, location, and extension of lesions |
| Age, comorbidity, immunosuppression |
| Mental status and adherence |
| Accessibility, cosmetic issues |
| History of skin cancer and prior treatments |
| Treatment cost |
| Patient preferences |
| Treatment-related factors27 |
| Availability, efficacy, safety, tolerance, and long-term outcomes |
| Physician-related factors |
| Experience with the procedure |
| Preferences |
AD_965_conv_Tab5-7.
Single or isolated lesions should be destroyed before they progress to invasive SCC (level of evidence 4, grade D√ recommendation).
The efficacy and safety of destructive therapies are difficult to assess because very few controlled clinical trials have been done, they have enrolled only a small number of patients, or none have been done for some treatments.8
None of the destructive therapies are useful for treating field cancerization. All but cryotherapy require local anesthesia and can leave scars or affect pigmentation.51 These procedures are generally easy to perform, rapid from the physician's point of view, and effective for treating isolated AKs. However, there is little standardization.
Surgical ExcisionSurgical removal of an AK is not routine and is undertaken only when there is suspicion of invasive SCC or lesions are recurrent. Surgery facilitates examination of the lesion by a pathologist to confirm the diagnosis. However, surgery is not indicated if multiple lesions are present.51
CryotherapyWorldwide, and particularly in Spain, cryotherapy is the procedure used most often because it is easy and fast, acceptable to patients, and inexpensive.52 The great problem with this method, however, is that little has been done to standardize the procedure (with regard to duration of application, intensity, frequency, temperature, etc.) and the recurrence rate is high—84.8% in a recent study in which histopathologic evaluation was performed.53 Because cryotherapy is a nonspecific procedure, it destroys both normal and atypical cells by disruption, separating the epidermis from the dermis.8 The efficacy of liquid nitrogen cryotherapy has been demonstrated by several studies that report cure rates of 67% to 99%; the cure rate is higher with longer application of the dry ice.54–56
Shave Excision or CurettageCurettage can be used alone or along with electrocoagulation. This procedure harvests tissue for pathology, although it is impossible to confirm whether there is tumor invasion of the margins. Curettage is particularly useful for treating a single AK or a small number of lesions, especially when they are hyperkeratotic, as when a cutaneous horn develops; generally the base is also treated by electrocoagulation.
Topical TreatmentTopical treatments are preferable to destructive ones in patients with multiple AKs and in cases with evident field cancerization, because these options treat both the lesion and the field26 (level of evidence 4, grade D recommendation).
These treatments offer several advantages over destructive therapies. By treating both lesion and field, the topical medication eliminates new visible AKs and subclinical ones as well as clones of cells that will become AKs.57 If field cancerization is treated, therefore, the intervals between treatment sessions can be lengthened and progression to invasive SCC prevented.27
An important consideration is that topical treatments for field cancerization are compatible with concomitant or sequential use of any of the treatments directed at particular lesions.27 We will now describe the mechanisms of action of the topical treatments listed in Table 5.
Principal Characteristics of Topical Treatments.
| Diclofenac 3% gel combined with 2.5% hyaluronic acid | |
| Application | Apply on the skin twice daily. |
| The amount needed depends on lesion size. Treatment usually lasts 60 to 90 days. The peak effect has been observed when the duration of treatment approaches the maximum time allowed. | |
| Complete clearing of lesions (or optimal therapeutic effect) may come as long as 30 days after end of treatment.91 | |
| Response rates | Around 85% (target lesion number score, ≥ 75%). |
| Complete clinical remission in 41.50% of patients after 90 days of treatment with 0.5 g twice daily.92,93 | |
| The efficacy of a single treatment cycle (90 days) with sodium diclofenac 3% gel has been maintained 1 year after end of treatment in most patients.92 | |
| Safety | Tolerance is very good. Adverse effects are confined to the skin, are mild to moderate, and consist of slight reddening, pruritus, dryness, and dysesthesia. Photoallergic reactions and contact dermatitis are rare.94,95 |
| High-risk patients | Complete clearing of lesions in 41% to 50% of organ transplant recipients using diclofenac 3% has been reported, demonstrating that this gel formulation is an efficient, well tolerated treatment for multiple AKs and that it prevents invasive SCC in these high-risk patients.87,96 |
| Imiquimod | |
| Application | Several different imiquimod 5% regimens have been used. The most common ones are application once a day, 3 times per week for 4 weeks or 2 to 3 times per week for 16 weeks.97 |
| Response rates | Cure rates between 45% and 84% have been reported with applications 2 to 3 times per week for 12 to 16 weeks.39,98 |
| One-year recurrence rate of 10%, 2-year recurrence rate of 20%.39,99 | |
| For a short course100–102 (3 applications per week for 4 weeks in 2 cycles separated by 4 weeks), complete clearance was observed in around 54% to 69% of patients (or 61% to 80% after 2 cycles). | |
| A meta-analysis of 5 studies (1293 patients) of a regimen of 3 applications per week for 12 to 16 weeks showed complete clearance in 50% of patients (vs 5% with placebo).80 | |
| Safety | Serious rash of variable and unpredictable intensity, different in every patient, but characterized by erythema and erosions, exudations and crusting, with pain and itching. Treatment of a very large area may cause fever, flu symptoms, and headache.97 |
| Treatment may have to be interrupted if intense local inflammatory reactions develop or the treatment site becomes infected. | |
| Good cosmetic results; skin quality maintained.103 | |
| High-risk patients | Imiquimod 5% treatment for AK in solid-organ (liver, kidney, heart) transplant recipients has been studied; histologic cures were reported in 18 of 29 patients (62.1%) on the active treatment and 0 of 14 patients (0%) on placebo.86 |
| PDT | |
| Application | To optimize treatment, first scrape the superficial keratin away. Then apply a photosensitizer and keep the area covered with an occlusive dressing for 3 h before illuminating with high-intensity red light. |
| Numerous protocols involving different photosensitizers, incubation times, and light sources have been studied, yet the optimal levels of illumination, wavelengths, and dose for treating AK remain to be determined104,105 | |
| PDT is mainly indicated for multiple superficial, confluent AKs or for AKs located in areas that heal with difficulty. | |
| Response rates | Response rates around 70% to 80% after 1 cycle, rising to 90% after 2 cycles.49 |
| A recent trial comparing 2 photosensitizers showed that PDT was effective for 45% with δ-aminolevulinic acid and 36% with methyl aminolevulinate at 12 months. | |
| A recurrence rate of 19% 1 year after treatment has been reported.106 | |
| Safety | As photosensitizers for PDT are not selective, treatment efficacy is reduced and hypersensitivity reactions to light may occur during the day. Penetration is limited (3–4 mm), and there may be pain during and after treatment.8,107,108 PDT requires investment of health care resources and is costly; trained staff is required.109 |
| About 60% of patients have local reactions at the site of illumination attributable to phototoxicity or site preparation (scraping the lesion). | |
| The most frequent side effects are local pain during treatment, lasting until the next day, and a certain degree of redness and edema at the treated site. However, the cosmetic outcome is excellent.105,109,110 | |
| 5-FU | |
| Application | Twice daily for 2–4 weeks8 |
| Response rates | Clinical cure rates range from 50% to 96%, with recurrence rates around 55%.88,90,111–114 |
| A recent study reported clinical cure rates of 96% (histologic cure, 67%) and a sustained clearance rate of 54% of initially treated lesions; sustained clearance of the total field in 33%.88 | |
| Safety | 5-FU induces a strong inflammatory reaction with itching, pain, erythema and other signs of varying degrees of intensity.8 The inflammation can be significant, sometimes leading patients to change their pattern of use of the cream or to interrupt treatment before the prescribed course is completed.90 |
| Phototoxic reactions and contact dermatitis have been described.89 Cancer patients who have dihydropyrimidine dehydrogenase deficiency are at higher risk of serious toxic reactions, with symptoms that include diarrhea, stomatitis, mucositis; myelosuppression; or neurotoxicity; on rare occasions the patient has died from these effects.115 | |
Abbreviations: 5-FU, 5-fluorouracil; OMIT OMIT; OMIT OMIT OMIT OMIT AK, actinic keratosis; PDF, photodynamic therapy. OMIT OMIT.
The combination of diclofenac 3% in 2.5% hyaluronic acid gel is a nonsteroidal anti-inflammatory drug formulation that inhibits cyclooxygenase 2 (COX-2), thus interfering with the upregulation of the arachidonic acid cascade and prostaglandin production. The resulting anti-inflammatory effect is derived from suppression of prostaglandin E2 (PGE2) production of immune-regulatory lymphocytes, T- and B-cell proliferation, and the cytotoxic activity of natural killer cells.
Nonsteroidal anti-inflammatory drugs also inhibit neoplastic cell proliferation by inducing apoptosis, and they reduce the upregulation of vascular endothelial growth factor, thereby inhibiting tumor angiogenesis by activating peroxisome proliferator-activated receptor gamma, decreasing cancer cell proliferation.8,58–63 The 2.5% hyaluronic acid that accompanies the diclofenac 3% helps transport the active ingredient and retain it in the epidermis, facilitating the effect of the NSAID.59 One study found that the formulation reduced levels of prostaglandin E2 in SCC cell lines sensitive to apoptosis (SCL-II, SCC-12, SCC-13), while the PGE2 and COX-2 remained undetectable in cells resistant to apoptosis (SCL-I).62
Imiquimod 5%Imiquimod is an immune response modifier. An agonist of toll-like receptor (TLR-7), imiquimod has antineoplastic and antiviral64 effects and stimulates both the innate and acquired immune response by means of TLR-7 activation of interferon and nuclear factor κB in monocytes and dendritic cells.
PDTPDT selectively destroys atypical keratinocytes (depth of penetration 3–4 mm) through light activation of a photosensitizer in the presence of oxygen, as noted in the European guideline.8 Metabolically active cells, such as neoplastic cells, take up more of the photosensitizer than normal cells. Under artificial light of a certain wavelength, the photosensitizer generates reactive oxygen species, causing photochemical and photothermal effects on the irradiated tissue. Precursors of protoporphyrin IX are used as photosensitizers. The most commonly selected agents are δ-aminolevulinic acid and its derivatives, such as the lipophilic agent methyl aminolevulinate.
5-Fluorouracil 5%As noted in the European guideline,8 5-fluorouracil (5-FU) 5% is a chemotherapeutic antimetabolite that interferes with DNA and RNA synthesis by blocking the methylation reaction of deoxyuridylic acid to thymidylic acid. Cell proliferation is prevented and cell death occurs in the absence of DNA synthesis, particularly in fast-growing dysplastic cells.8 Topical 5-FU is not currently available in Spain.
New Treatment OptionsNew drugs and formulations of existing ones have recently appeared on the market, expanding the therapeutic arsenal (Table 6).
Principal Characteristics of New Treatment Options.
| 5-FU 0.5% combined with 10% salicylic acid | |
| Application | Dosage is for adults, including patients of advanced age. |
| Apply the product once a day until complete clearance is achieved, or up to a maximum of 12 weeks116 | |
| Response rates | In a pilot study with this formulation, 77% of AKs cleared after 84 days of treatment.117 |
| Safety | Mild to moderate irritation and inflammation at the site of application.116 |
| Imiquimod 3.75% | |
| Application | This formulation offers advantages over imiquimod 5%. Larger areas (> 25 cm2) of skin, such as the entire scalp or whole face, can be treated. |
| Furthermore, the treatment regimen is simpler and shorter with the lower-dose cream (3.75%): the regimen lasts 6 weeks (application twice daily for 2 weeks, then a rest period of 2 weeks, followed by application once a day for 2 weeks).8 | |
| Response rates | Complete clearance of all AKs (including lesions detected during treatment and “subclinical” ones) in 36% of patients (vs 6% of placebo-treated patients).66,67 |
| Partial clearance was achieved (> 75% reduction in AKs) in 59% of patients treated with imiquimod 3.75% (vs 23% treated with placebo).66,67 | |
| Ingenol mebutate | |
| Application | The gel is supplied in single-use tubes and should be applied to a maximum area of 25 cm2 (for example an area 5cm×5cm).69 |
| The product should not be applied on broken or damaged skin, near the eyes, inside the nose or ears, or on the lips. Nor should it be used on skin that has not recovered from a previous treatment.69 | |
| Response rates | Ingenol mebutate 0.015% applied for 3 days to treat facial or scalp AKs achieved complete and partial (≥ 75%) remission at 57 days in 42.2% and 63.9% of patients, respectively.118 |
| Rates of complete and partial remission for patients with localized lesions on the trunk and extremities treated with ingenol mebutate 0.05% for 2 days were 34.1% and 49.1%, respectively.69 | |
| Recurrence rates of localized AKs 12 months after treatment were 13.2% for sites on the trunk and extremities (ingenol mebutate 0.05%) and 12.8% for the face and scalp (ingenol mebutate 0.015%).69,119,120 | |
| Safety | The most commonly reported adverse reactions are local skin irritation with erythema, scaling, crusting, swelling, blistering or formation of pustules, erosion or ulcers.69 |
| Most patients (> 95%) experience 1 or more local reactions after use. The incidence of serious reactions is 29% for treatment on the face and 17% for treatment on the trunk or extremities.69 | |
| Resiquimod | |
| Response rates | Reported response rates after a single treatment cycle of differing concentrations (0.1%, 0.03%, 0.06% and 0.1%) applied once a day for 4 weeks were 40.0%, 74.2%, 56.3% and 70.6%, respectively; after 2 cycles the response rates were 77.1%, 90.3%, 78.1% and 85.3%.121 |
| The cure rates after 2 cycles were similar for the different concentrations in that study, but lower concentrations are better tolerated.121 | |
| Safety | Serious local adverse effects and flu-like symptoms developed in 0%, 3%, 12% and 13% of patients (for concentrations of 0.01%, 0.03%, 0.06% and 0.1%, respectively, applied once daily for 4 weeks).121 |
Abbreviations: 5-FU, 5-fluorouracil; SCC, squamous cell carcinoma; AK, actinic keratosis.
A new topical formulation of 5-FU 0.5% with 10% salicylic acid was recently approved and is already being sold in some European countries. This formulation boasts fewer side effects than 5-FU 5%. Salicylic acid is a keratolytic agent that has long been used to treat various skin diseases with a hyperkeratotic component. In combination with a low dose of 5-FU (0.5%) it can reduce the hyperkeratosis of AKs and enhance penetration of the principal ingredient.8
Imiquimod 3.75%Although imiquimod 3.75% is not currently available in Spain, it is indicated for the topical treatment of visible or palpable AKs on the face or scalp of immunocompetent adults, provided the lesions are neither hyperkeratotic nor hypertrophic; this option is appropriate in individuals for whom other treatments are contraindicated or considered less appropriate.65 The 3.75% formulation offers several advantages over the imiquimod 5% cream. First, it can be applied over larger areas of skin (> 25 cm2), such as the entire scalp or face. Second, even though the 3.75% formulation causes the same type of adverse effects as the 5% cream, in the usual sites of application, the incidence of these effects is lower (10.6% with the dose of the lower 3.75% concentration, 33% with the higher 5% concentration). Moreover, fewer patients treated with imiquimod 3.75% than patients treated with imiquimod 5% stop treatment because of adverse effects.66,67
Ingenol MebutateIngenol mebutate (ingenol-3-angelate, formerly PEP005) is a diterpene ester extracted and purified from the plant Euphorbia peplus.68 It was recently approved by the European Medicines Agency for the treatment of nonkeratotic, nonhypertrophic AK in adults.69 The mechanism of action is not fully understood but 2 functions for this substance have been demonstrated by means of in vivo and in vitro models: 1) local induction of cell death and 2) promotion of an inflammatory response characterized by infiltration of immunocompetent cells.69
ResiquimodResiquimod is an antagonist of TLR-7 and TLR-8.8 Its immunomodulatory effects are similar to those of imiquimod, but resiquimod activates both myeloid and plasmacytoid dendritic cells and also induces greater secretion of interleukin 12 and tumour necrosis factor.70
Other TreatmentsOral systemic retinoids, dermabrasion, chemical peeling, and carbon dioxide laser therapy are considered second-line or coadjuvant treatments and they should be considered for possible use in special circumstances8,71,72 (level of evidence 4, grade D√ recommendation).
Preventive therapy with oral retinoids is recommendable in combination with the topical treatment of field cancerization, at least in patients with a history of invasive SCC who have multiple AK lesions73 (level of evidence 4, grade D recommendation).
Solid-Organ Transplant Recipients and AKs at High Risk of ProgressionLong-term immunosuppression in solid-organ transplant recipients to prevent graft rejection increases the risk of infections and the development of precancerous and cancerous lesions; among them are AKs, which often have an atypical clinical presentation in these patients. The relative risk of AK is 250-fold higher in transplanted patients than in immunocompetent individuals.74 Multiple AKs are common in these patients (cumulative 5-year incidence, 35% to 40%), and there is greater risk of progression to invasive SCC as well as faster progression.75–77
For AKs at high risk of progression to invasive SCC, the physician should consider treatments that will facilitate pathologic diagnosis, although topical treatments can also be applied to increase efficacy78–83 (level of evidence 4, grade D recommendation). Particularly risky are lesions on the lips, ears, or around the eyes, as invasive SCCs in these locations have greater ability to metastasize.82,84 The possibility of metastasis should also be considered when AKs appear in patients who have a history of invasive SCC, have lesions that are secondary to ionizing radiation, are positive for the human immunodeficiency virus, or are solid-organ transplant recipients.27 In the last group, good results have been reported for application of diclofenac 3% and imiquimod 5%, so a promising approach is to combine medical treatment with destructive procedures that facilitate a pathologic diagnosis.78,85–87
Measures to prevent AK (extreme sun protection measures) are recommended for solid-organ transplant recipients; moreover, in view of the higher prevalence of AK in this group and faster progression to invasive SCC in comparison with immunocompetent individuals, these patients should also be examined periodically so that lesions can be detected and treated early42,75–77 (level of evidence 4, grade D recommendation).
Surgery is recommended in patients with AK that resists treatment or that recurs so that material for pathologic diagnosis can be obtained and invasive SCC ruled out.
ConclusionsBased on the social, epidemiologic, cultural, and climate characteristics in Spain, we can expect the prevalence of diseases like AK to rise and the demand for care to increase. Although a sun-safety culture is becoming better established, we must also cope with population aging and the reduction in atmospheric protection because of ozone layer depletion, making us more vulnerable to the effects of UV radiation.
In Spain, the treatment of AK usually falls to the dermatologist, who uses ablative therapies that target lesions without regard for field cancerization. The therapeutic arsenal has expanded, mainly through the development of topical treatments for AK that are highly useful against field cancerization. We therefore believe experts need consensus-based recommendations (Table 7) that facilitate their choice of the best treatment options for individual patients. The choice will be based on the features of the treatment, the type of lesion, the presence or absence of field cancerization, and the patient's preferences. 5-FU can be applied on multiple lesions twice a day for 2 to 4 weeks; the rate of initial clearance of lesions is high with this cream, but the 12-month recurrence rate is also high.88 It can cause such adverse effects as serious rashes, phototoxicity, or contact dermatitis that can lead patients to stop treatment.89 The formulation combining 5-FU 0.5% with 10% salicylic acid, however, has a better safety profile.90 Treatment with imiquimod 5% is associated with a lower recurrence rate, and the clearance rate 12 months later is high. Maintenance of skin quality at the end of treatment with imiquimod 5% is also good. Some patients have to abandon imiquimod use, however, when they develop serious rashes, although this drug is generally reasonably well tolerated.88 The imiquimod 3.75% cream can be applied over larger areas of skin, such as the scalp or face, although it is indicated only for visible or palpable AKs that are neither hyperkeratotic nor hypertrophic, or it can be used when other topical treatments are contraindicated or inappropriate.65 Diclofenac 3% in 2.5% hyaluronic acid gel can also be applied over large areas, the treatment can be continued for 60 to 90 days,91 and tolerance is excellent. Ingenol mebutate, on the other hand, is only applied for 3 days and the results are not assessed until 8 weeks later.69 This formulation has only recently been approved in Spain, and there is little clinical experience with it as yet. Finally, PDT is an excellent choice but has the drawbacks of high cost and scarce availability; moreover, patients must travel to clinics where it can be administered, with consequent loss of work time.
Recommendations.
| Recommendation | References With Supporting Evidence | Level of Evidence / Grade of Recommendation | Reference With Prior Publication of the Recommendation | |
| 1 | AKs are in situ SCC lesions at low risk of progressing to invasive SCC. They present as slightly erythematous, scaly lesions in areas of chronically sun-damaged skin. | Stockfleth et al.8 | 4D | Stockfleth et al.8 |
| 2 | Solid-organ transplant recipients should use extreme sun protection measures to prevent AKs from developing. Likewise, they should be examined for AKs periodically so lesions can be detected early and treated. AK is more prevalent in these patients and progression to invasive SCC occurs more rapidly than in immunocompetent individuals. | Ulrich et al.,42 Fuente et al.,75 Ferrandiz et al.,76 Johnson et al.77 | 4D | Ulrich et al.42 |
| 3 | AKs should be biopsied if they are inflamed, indurated, ulcerated, bleeding, or measure > 2 cm in diameter; a biopsy is also necessary if lesions are growing rapidly, do not clear after adequate treatment, or recur after successful treatment. The main purpose of biopsy is to rule out invasive SCC and to differentiate AKs from other types of lesions when the clinical diagnosis is in doubt. | Stockfleth et al.8 | 4D | Stockfleth et al.8 |
| 4 | AKs should be considered in situ SCCs because they display proliferation of atypical keratinocytes with the same changes as those seen in invasive SCCs: nuclear pleomorphism and hyperchromias, mitotic figures, and loss of polarity. | Ackerman,23 Ackerman et al.,24 Guenthner et al.,25 Jorizzo et al.,26 Stockfleth et al.27 | 4D | Stockfleth et al.8 |
| 5 | AKs should be treated because they are in situ SCCs that may become invasive SCCs. | Massa et al.,6 Stockfleth et al.,8 Braakhuis et al.22 | 4D | Stockfleth et al.8 |
| 6 | Whenever possible field cancerization should be targeted so that subclinical lesions are treated to prevent them from progressing to clinically visible ones and invasive disease. | – | 4D√ | – |
| 7 | Destructive therapies should be applied to solitary AKs or whenever invasive SCC is suspected. | – | 4D√ | – |
| 8 | Topical treatments are preferable to destructive ones in patients with multiple AKs or evident field cancerization because topical formulations treat both the visible lesions and the field. | Jorizzo et al.26 | 4D | – |
| 9 | Orally administered systemic retinoids, dermabrasion, chemical peelings, and CO2 laser treatments are second-line or coadjutant therapies. They should be considered for use in special circumstances. | Stockfleth et al.,8 Moriarty et al.,71 Yu et al.72 | 4D√ | – |
| 10 | The physician should assess the relevant factors and select the treatment that is most appropriate for the individual patient. It is important to note that a destructive treatment may need to be combined with a topical treatment in some circumstances. | – | 4D√ | – |
| 11 | The use of oral retinoids for cancer prevention in addition to topical treatment of field cancerization is advisable, at least in patients with a history of invasive SCC or with multiple AKs. | Carneiro et al.73 | 4D | – |
| 12 | In case of AKs at high risk of progression to invasive SCC, consider treatment procedures that will allow for a pathologic diagnosis, even if topical treatments are also being prescribed to enhance efficacy. | Del Rosso,78 Ehrig et al.,79 Hadley et al.,80 Perrett et al.,81 Rowe et al.,82 Serra-Guillen et al.83 | 4D | – |
| 13 | Primary and secondary preventive measures are the best way to manage AK and these measures are absolutely essential for high-risk patients. | Stockfleth et al.,8 Ulrich et al.,42 Ulrich et al.,43 Berman et al.,122 Schwartz et al.123 | 1A | – |
| 14 | AKs are considered a sign of chronic sun damage and they identify patients at higher risk of developing nonmelanoma skin cancer. | Chen et al.41 | 4D√ | – |
| 15 | The patient with AKs should be examined periodically, to promote early diagnosis, treatment and sun protection behaviors. | Stockfleth et al.,27 Schmitt et al.,124 Fenske et al.,125 Strickland et al.,126 Pandey et al.127 | 4D | – |
Abbreviations: SCC, squamous cell carcinoma; AK, actinic keratosis.
Given the unceasing rise in the incidence and prevalence of skin cancers, including AK, and considering that the primary care doctor is the first to see the patient, there is no doubt that this physician will play an important role in the diagnosis and treatment of AK. A primary care doctor's duty goes beyond recognizing AKs when the patient asks about them; it is also necessary to look for them actively because patients who visit with other complaints may be unaware of the nature of visible lesions that are asymptomatic. The primary care doctor's ability to find cases, treat incipient lesions, and refer patients to the dermatologist (when lesions are doubtful, require ablation, or there is field cancerization) will be crucial in the effort to lower the incidence of AK and invasive SCC.
Implementing recommendations based on systematic review of the available evidence will not only help reduce variability in clinical practice between specialists but will also facilitate more effective use of health care resources, bringing the patient to the point of diagnosis and appropriate treatment as quickly as possible. The periodic updating of reviews of the literature on the diagnosis and treatment of AK provides information for improving patient care, encourages greater consistency in decision making, and promotes awareness among all involved health care providers.
FundingLaboratorios Almirall S.A. has sponsored the collaboration necessary to produce this paper, without interfering in the decisions of the expert group.
Conflicts of InterestCarlos Ferrándiz, in relation to AK, has received fees for speaking at conferences, collaborating with clinical trials, and consulting or otherwise assisting with research from Almirall, Leo-PharmayIsdin. Eduardo Fonseca Capdevila has participated in continuing professional development activities and clinical trials funded by Abbott, Almirall, Celgene, FaesFarma, Janssen, MSD, and Pfizer. Amaro García Diez has served as a consultant, given talks, assisted with research, and received grants from Almirall, Isdin, Serono, Abbott, Abbie, Leo Pharma, Pfizer, and MSD. Carlos Guillén Barona has received consulting or speaking fees and funds for collaboration with clinical trials, consulting, or conducting research from Almirall, Leo-Pharma, 3M, Meda, and Galderma; all such funding was related to nonmelanoma skin cancer. Isabel Belinchón Romero has collaborated with research, conferences, and training courses sponsored by Abbott, MSD, Pfizer, Janssen, Leo Pharma, and Almirall. Pedro Redondo Bellón has received fees from Almirall, MSD, Galderma, and Allergan. José Carlos Moreno Giménez has received grants from the following laboratories or collaborated with research, conferences, and publications they sponsored: MSD, Pfeizer, Leo-Pharma, GSK, Galderma, Amirall, Abbot, and Janssen. Rosa Senan has received consultancy fees from Almirall and conference funding from GSK.
Please cite this article as: Ferrándiz C, Fonseca-Capdevila E, García-Diez A, Guillén-Barona C, Belinchón-Romero I, Redondo-Bellón P, et al. Adaptación española de la Guía europea para la evaluación y tratamiento de la queratosis actínica. Actas Dermosifiliogr. 2014;105:378–393.