Solar urticaria (SU) is a rare chronic inducible urticaria. The management of SU is challenging, with only one small clinical trial and case series reporting variable outcomes with omalizumab.1,2
We conducted a single-center, retrospective case-control study of patients with SU on omalizumab. The primary endpoint was to find clinical, photo-biological, or serological differences that could help us predict which patients have more chances of resorting to omalizumab. The clinical response was evaluated using the Urticarial Control Test (UCT). Complete clinical response (CCR) was defined as 16 points on the UCT.3 We used the SPSS statistics software, and Fisher's exact test and the Mann–Whitney U test for qualitative and quantitative variables, respectively. All patients underwent baseline photo-testing according to the protocols drafted by the AEDV Spanish Photobiology Group.4
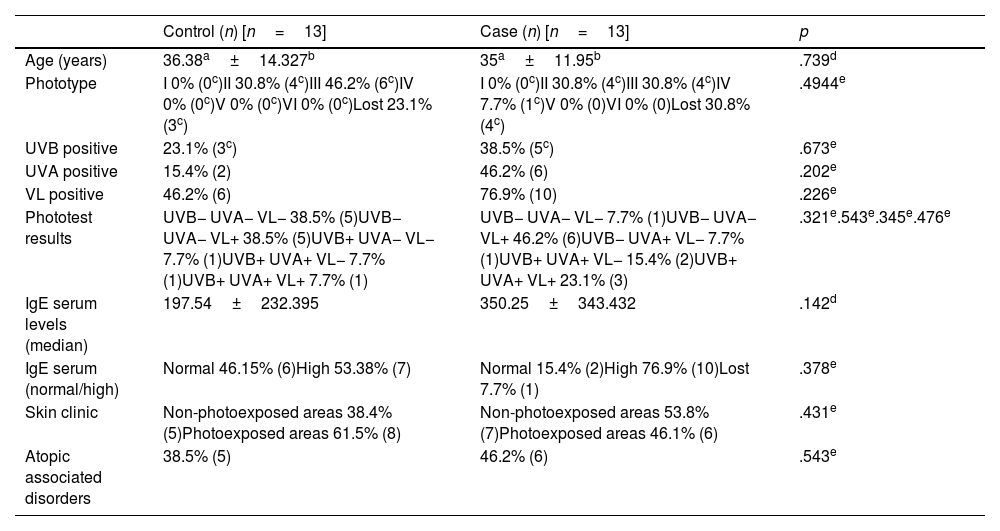
A total of 59 patients with SU were seen from January 2015 to December 2021. Thirteen patients (22%) needed treatment with omalizumab after failed antihistamine therapy (case group). Thirteen out of the remaining 46 patients were randomly selected (control group) by the computer program, Random Generator. The mean age was similar, 36.4 years in the case group vs 35 years in the control one. Similar results were found in both groups regarding phototype, history of atopy and clinical presentation. We obtained 3 important results: first, the most widely implicated light spectrum was visible light in both groups, but on a higher rate in the case group (76.9% vs 46.2%). Regarding phototest results, the case group had more patients with positive phototests in all wavelengths (23.1% vs 7.7%). In contrast, the control group had more patients with negative phototests (38.5% vs 7.7%). Finally, we saw differences in IgE levels: 77% of the patients in the case group showed abnormal IgE levels with mean levels of 350IU/mL vs 53.8% with mean levels of 197IU/mL in the control group. None of the differences mentioned above were statistically significant (Table 1).
Clinical and epidemiological characteristics of control and case patients.
| Control (n) [n=13] | Case (n) [n=13] | p | |
|---|---|---|---|
| Age (years) | 36.38a±14.327b | 35a±11.95b | .739d |
| Phototype | I 0% (0c)II 30.8% (4c)III 46.2% (6c)IV 0% (0c)V 0% (0c)VI 0% (0c)Lost 23.1% (3c) | I 0% (0c)II 30.8% (4c)III 30.8% (4c)IV 7.7% (1c)V 0% (0)VI 0% (0)Lost 30.8% (4c) | .4944e |
| UVB positive | 23.1% (3c) | 38.5% (5c) | .673e |
| UVA positive | 15.4% (2) | 46.2% (6) | .202e |
| VL positive | 46.2% (6) | 76.9% (10) | .226e |
| Phototest results | UVB− UVA− VL− 38.5% (5)UVB− UVA− VL+ 38.5% (5)UVB+ UVA− VL− 7.7% (1)UVB+ UVA+ VL− 7.7% (1)UVB+ UVA+ VL+ 7.7% (1) | UVB− UVA− VL− 7.7% (1)UVB− UVA− VL+ 46.2% (6)UVB− UVA+ VL− 7.7% (1)UVB+ UVA+ VL− 15.4% (2)UVB+ UVA+ VL+ 23.1% (3) | .321e.543e.345e.476e |
| IgE serum levels (median) | 197.54±232.395 | 350.25±343.432 | .142d |
| IgE serum (normal/high) | Normal 46.15% (6)High 53.38% (7) | Normal 15.4% (2)High 76.9% (10)Lost 7.7% (1) | .378e |
| Skin clinic | Non-photoexposed areas 38.4% (5)Photoexposed areas 61.5% (8) | Non-photoexposed areas 53.8% (7)Photoexposed areas 46.1% (6) | .431e |
| Atopic associated disorders | 38.5% (5) | 46.2% (6) | .543e |
The statistical program SPSS, Fisher's exact test and the Mann–Whitney U test were used for qualitative and quantitative variables, respectively.
On the other hand, we studied the course of the disease of 10/13 patients who had been on a 1-year course of omalizumab. Mean follow-up was 35 months. Nine of the patients achieved CCR with 300mg every 4 weeks. Among them, 4 patients achieved CCR with de-intensification dosage (300mg every 6 weeks). De-intensification occurred 6 months (minimum) after achieving CCR and after a whole summer on therapy. Before de-intensification, the 4 patients were treated for 6, 12, and 18 months, respectively, with omalizumab 300mg every 4 weeks. None of the patients achieved CCR with omalizumab 300mg every 8 weeks. No adverse events were reported, and none of the patients discontinued treatment.
We have found more photosensitivity and higher IgE levels in patients on omalizumab than in the control group. We should mention that the IgE levels reported in the case group were slightly higher than those seen in a large serie of patients with SU in the scientific medical literature currently available.5 The rates of CCR were similar to those reported in former studies, but we obtained higher rates of CCR with de-intensified dosage (300mg of omalizumab every 6 weeks).6
Out study has some limitations such as its retrospective design and limited number of patients, which complicates finding statistically significant differences. Prospective, multicenter studies would be necessary to verify our results.
Omalizumab could be the second therapeutic step in patients with SU, with similar dosage as chronic spontaneous urticaria, with the possibility of intensification/de-intensification.
Conflict of interestsThe authors declare that they have no conflict of interest.