Scabies is a highly prevalent parasitic disease that affects almost 200 million people and can have a serious socioeconomic impact.1,2 Clinical diagnosis is usually straightforward, but in some cases diagnostic confirmation can be difficult. Dermoscopy can be helpful to identify the delta wing sign (a brownish triangular structure at the end of wavy whitish lines) and grooves created in the skin by the mite, and to guide sample collection for cytology (Muller test). In vivo reflectance confocal microscopy (RCM) has recently been described as a useful technique for the diagnosis of scabies and various parasites.3 We describe a case of scabies that had been treated with multiple therapies but nonetheless persisted, as was confirmed by RCM.
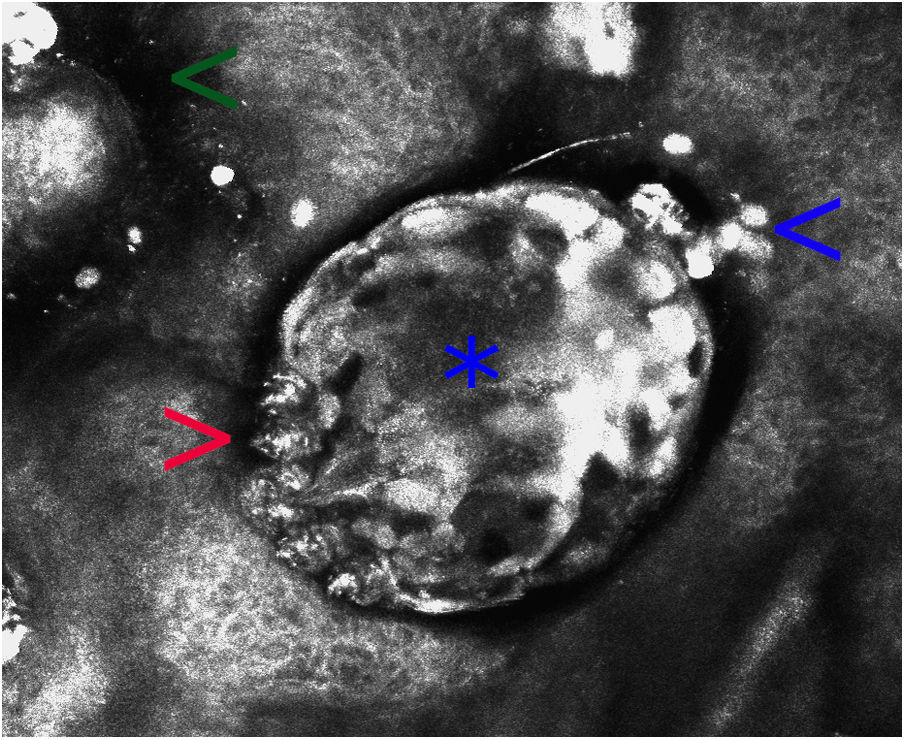
The patient was a 26-year-old heterosexual man with a history of HIV infection who was receiving a highly active antiviral treatment, which provided good immune control (undetectable viral load; 503 CD4 lymphocytes/µL). He was seen by emergency health services for itching that had begun 4 months earlier. Scabies was clinically suspected based on the predominantly nocturnal pruritus and the presence of multiple excoriated lesions on the wrists, and the patient was prescribed 5% permethrin cream, oral antihistamines, and, subsequently, topical corticosteroids. The patient applied the scabicide on 2 occasions but the pruritus persisted and he was referred to the dermatology service. Physical examination revealed multiple burrow tracks on the wrists and hands. The delta wing sign was observed on dermoscopy. A skin scraping was taken from 2 burrow tracks on the left hand for cytology. The results were negative, showing no evidence of mites. Given the high clinical suspicion, RCM (VivaScope® 3000) of the burrow tracks on the right hand was performed, and clearly revealed the presence of the parasite (Fig. 1), scybala (feces), and nymphs. Intestinal peristalsis of the parasite was even visible in real time. The patient was prescribed daily baths with 0.03% potassium permanganate owing to the presence of exudative lesions; ivermectin (200 μg/kg [18-mg tablet] on days 0 and 10); and a new course of treatment with 5% permethrin cream. The itching gradually decreased after the second week, and complete resolution of the clinical picture was confirmed upon evaluation after 3 weeks.
Confocal reflectance microscopy image from a patient with scabies (field of view, 500 × 500 μm). An oval structure of variable refractivity corresponds to the body of Sarcoptes scabiei (asterisk). The legs (red arrow), smaller hyper-refractive ovoid structures corresponding to feces (blue arrow), and poorly defined hyporefractive areas corresponding to the mite furrow (green arrow) can be observed. No eggs or nymphs are visible in this image.
Diagnosis of scabies is based on clinical features and detection of the parasite, its eggs, or scybala on microscopic observation of skin scrapings taken from burrow tracks.1 However, the sensitivity of the Muller test is low. False negatives, as in the present case, are not uncommon and the procedure requires experience and time.4 Various molecular tests such as enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) have been recently developed for the detection of Sarcoptes scabiei, with good initial results, although these tests are not yet commercially available.4,5 RCM is a novel diagnostic technique in dermatology that enables the diagnosis of tumors and inflammatory and infectious diseases with high sensitivity and specificity.3 It is fast, painless, does not require contrast agent, and provides a horizontal view of the various layers of the skin with cellular resolution, without damaging the tissue. Disadvantages of RCM include its limited availability (due to its high cost), the need for specialized training to interpret the results, and its limited penetration of the skin (approximately 200–300 μm, which only allows observation to the level of the superficial dermis). On RCM S scabiei appears as an ovoid form with variable refractivity. The legs and even the digestive tract can be distinguished. Scybala are hyper-refractive and the eggs and mite furrow are hypo-refractive.3 In addition to enabling confirmation of the presence of the mite, RCM can also provide information on mite viability through in vivo observation of mite mobility and digestive tract peristalsis, and is thus a useful means of monitoring the therapeutic response.3,6 RCM studies have revealed that in Norwegian scabies the skin of the host can harbor an estimated 15.8 million mites and 7.2 million eggs.7 RCM allows easy visualization of other parasites, including Demodex folliculorum, as well as various mycoses.3
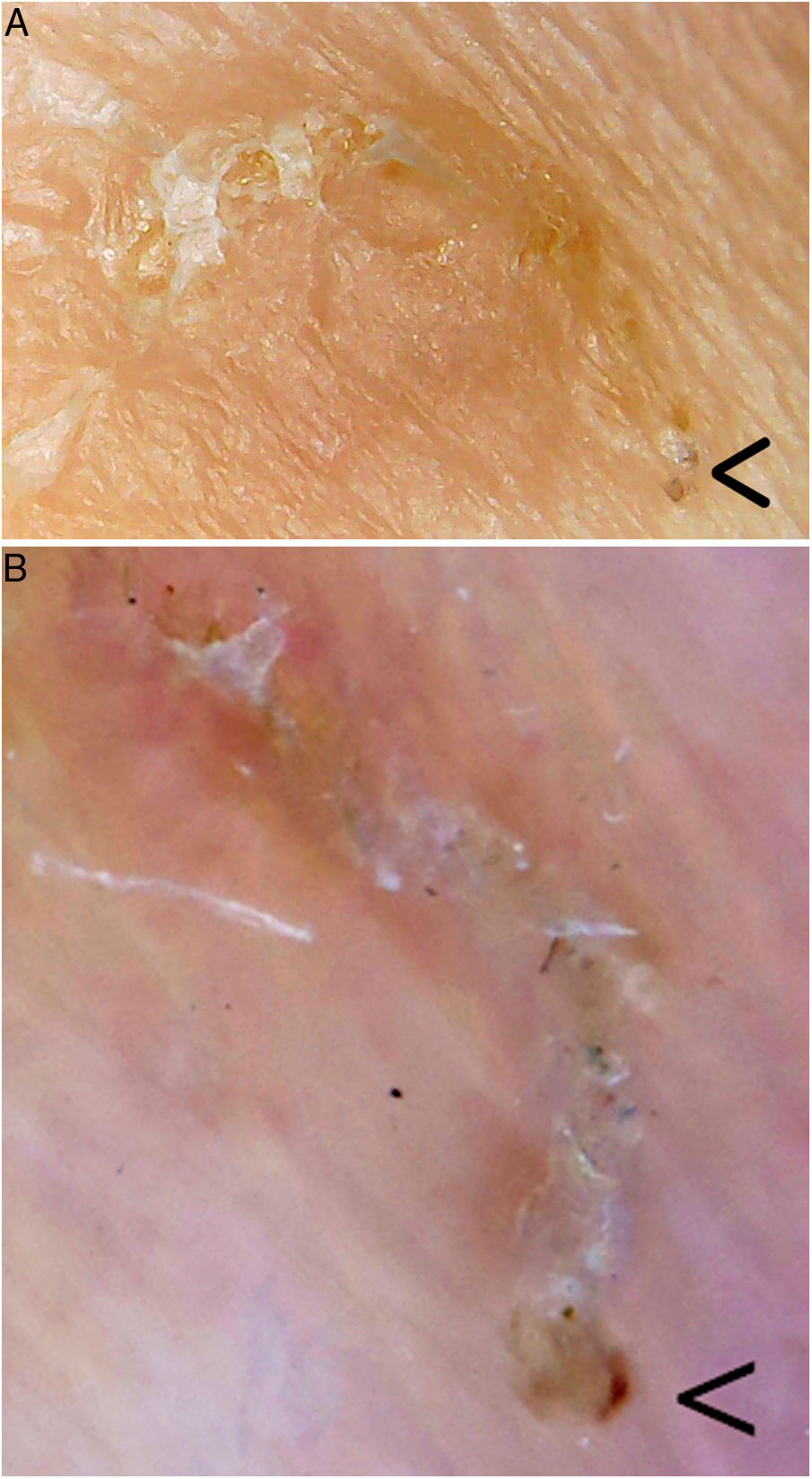
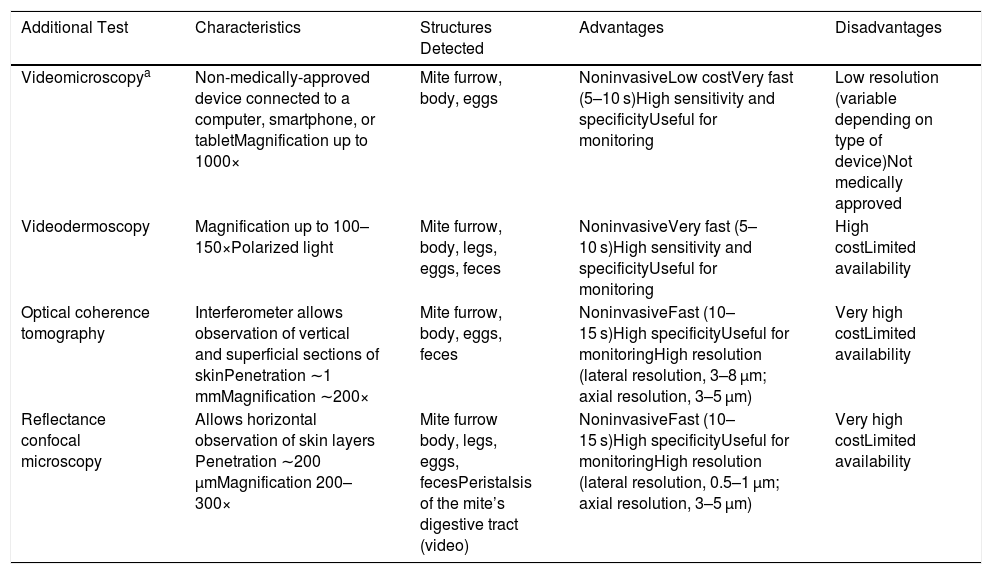
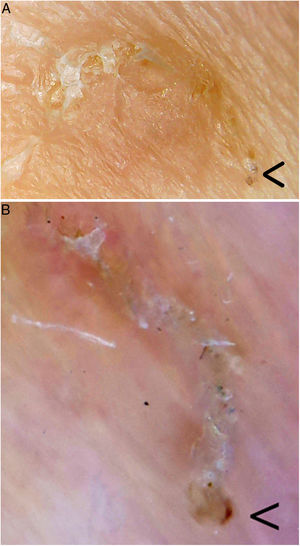
Videodermoscopy, videomicroscopy, and optical coherence tomography are other highly specific imaging techniques that have been recently used in the diagnosis of scabies (Table 1). These techniques enable rapid diagnosis without the need for tissue manipulation, thereby reducing the risk of contagion of healthcare personnel. Disadvantages of these approaches include their limited availability and the high cost of some of these devices.8 One exception is videomicroscopy, which can be performed using non-medically-approved devices used in jewelry, electronics, bricolage, and entomology, among other fields. Generally, a nonpolarized light is used and the device is connected directly or wirelessly to a computer, smartphone, or tablet. These devices are widely available for purchase online, and range in price from $20 to $50. Videomicroscopy is a cost-effective alternative and could revolutionize the diagnosis and follow-up of patients with scabies, especially in developing countries.9 In our practice we have used one of these devices (which cost less than €20) to identify mite furrows, and can confirm that it provides excellent magnification and resolution superior to dermoscopy (Fig. 2).
New Imaging Tests for the Diagnosis of Scabies
| Additional Test | Characteristics | Structures Detected | Advantages | Disadvantages |
|---|---|---|---|---|
| Videomicroscopya | Non-medically-approved device connected to a computer, smartphone, or tabletMagnification up to 1000× | Mite furrow, body, eggs | NoninvasiveLow costVery fast (5–10 s)High sensitivity and specificityUseful for monitoring | Low resolution (variable depending on type of device)Not medically approved |
| Videodermoscopy | Magnification up to 100–150×Polarized light | Mite furrow, body, legs, eggs, feces | NoninvasiveVery fast (5–10 s)High sensitivity and specificityUseful for monitoring | High costLimited availability |
| Optical coherence tomography | Interferometer allows observation of vertical and superficial sections of skinPenetration ∼1 mmMagnification ∼200× | Mite furrow, body, eggs, feces | NoninvasiveFast (10–15 s)High specificityUseful for monitoringHigh resolution (lateral resolution, 3–8 µm; axial resolution, 3–5 μm) | Very high costLimited availability |
| Reflectance confocal microscopy | Allows horizontal observation of skin layers Penetration ∼200 μmMagnification 200–300× | Mite furrow body, legs, eggs, fecesPeristalsis of the mite’s digestive tract (video) | NoninvasiveFast (10–15 s)High specificityUseful for monitoringHigh resolution (lateral resolution, 0.5–1 µm; axial resolution, 3–5 µm) | Very high costLimited availability |
Mite furrow. The parasite is located in the bottom right corner (arrow) in each image. A, Videomicroscopy. The magnification used is between 60× and 100×; it is difficult to establish the true magnification as the device used (Jiusion 40–1000× microscope) is not medically approved. B, Dermoscopy (8× magnification; DermLite DL200, 3 Gen). Videomicroscopy provides greater magnification and resolution.
A range of imaging tools that facilitate the diagnosis of scabies have recently emerged. RCM is a rapid, painless, highly sensitive and specific additional technique that can help establish diagnosis in atypical or doubtful cases of scabies.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morgado-Carrasco D, Fustà-Novell X, Rizo D, Alsina M. Nuevas tecnologías para el diagnóstico de la escabiosis: sarna de evolución tórpida con diagnóstico confirmado por microscopia confocal de reflectancia. Actas Dermosifiliogr. 2021;112:271–273.