The increase in the number of diabetic patients who used glucose sensors has led to more frequent findings of local skin reactions caused by these devices. Affected patients are evaluated by dermatologists, endocrinologists, and pediatricians. Since not all hospitals have cutaneous immunoallergy units, the specialist must be aware of the peculiarities of this new contact dermatitis “epidemic”. Recent years have seen the publication of numerous articles on skin reactions caused by glucose sensors.
The first sensor marketed (not funded at the time) was FreeStyle Libre (Abbot Laboratories). Subsequent funding of the device led to a considerable increase in its use by patients with type 1 diabetes mellitus (DM). Through enhanced control of blood glucose, the device changed the lives of many patients with diabetes mellitus, enabling them to lead an almost normal life, in terms of both their social setting and sporting activities. However, cutaneous inflammatory reactions at the application site have not gone unnoticed, and their prevalence has been estimated at between 3.8%1 and 8.4%.2 The fact that the FreeStyle Libre glucose sensor is occluded for 14 days has 2 major implications. First, the sensor remains occluded for a long period, and, second, it is necessary to apply a material that enables the device to be used for such a long period. This is exactly where the main allergen to date, isobornyl acrylate (IBOA; CAS 5888-33-5), comes into play. Many published articles show that IBOA is the main culprit behind allergic contact dermatitis to FreeStyle Libre®.3 A positive reaction to IBOA was observed in 83.3% of pediatric patients studied because of cutaneous reactions to the device.4 Some authors have proposed changing the glucose sensor to Eversense (Roche)5 or Dexcom (Medtronic)6 in order to resolve the problem of skin reactions. However, this proposal is “problematic” for a number of reasons. First, to date, these glucose sensors are not publicly funded in Spain and are very expensive. As an example, Dexcom G5 costs around Є200 every 7 days, which is when the sensor is changed, thus preventing the device from being universally affordable. Second, the use of these devices is not exempt from skin reactions, which are initially irritant, although for now, we do not know whether they contain a potentially sensitizing allergen. However, patients who come to my eczema clinic (diagnosed with contact dermatitis to IBOA) mentioned that they had received a new version of the FreeStyle Libre device. Tolerance to the device was good for the first 14 days, and it seems that the composition of the adhesive has been modified to eliminate IBOA, since the patients were unable to tolerate the sensor for more than 48 hours owing to allergic contact dermatitis. Oppel et al.7 recently published an interesting article in which they analyze the new FreeStyle Libre 2 sensor, which does not contain IBOA. The allergens that could be associated with sensitization include 2,6-di-tert-butyl-4-cresol (butylated hydroxytoluene), which is now included in the new adhesive. Therefore, it should form part of the specific allergen series that we use for patch testing in patients who develop contact dermatitis after using FreeStyle Libre 2.
Other allergens associated with glucose sensors are colophony and Abitol,8 sesquiterpene lactone mix,9 and N,N-dimethylacrylamide.10 N,N-dimethylacrylamide has been shown to be present in the adhesive of FreeStyle Libre, and after IBOA, it is the second most frequent allergen associated with contact dermatitis to this device. N,N-dimethylacrylamide is not currently commercially available for patch testing, although it may be in the future. It can therefore form part of the group of allergens to be assessed in patients with contact dermatitis to glucose sensors. N,N-dimethylacrylamide can be purchased directly from Sigma-Aldrich, where it is prepared in the laboratory (or hospital pharmacy) in an appropriate vehicle, such as petrolatum. It must be borne in mind that N,N-dimethylacrylamide is combined with another substance, hydroquinone; therefore, when assessing this allergen, hydroquinone should be tested individually in order to prevent diagnostic errors.
The fact that some patients who are sensitized to IBOA experience positive reactions to sesquiterpene lactone mix could be explained by the isomerization of α-pinene to camphene; binding to a methylene group, a hydrogen, and 2-dioxygens leads to the structure of IBOA.
Insulin pumps, which constitute another medical device directly related to glucose sensors,11 can also cause allergic contact dermatitis, with the main culprit allergens being IBOA, N,N-dimethylacrylate, and 2-ethyl-cyanoacrylate.
Patients who use glucose sensors clearly require special care of the skin where the device is applied. In this sense, there have been reports of various materials with a barrier function, such as hydrocolloid patches12 and Tegaderm Advanced dressings.13 While these approaches work for some patients, my experience indicates that they are not a long-term solution.
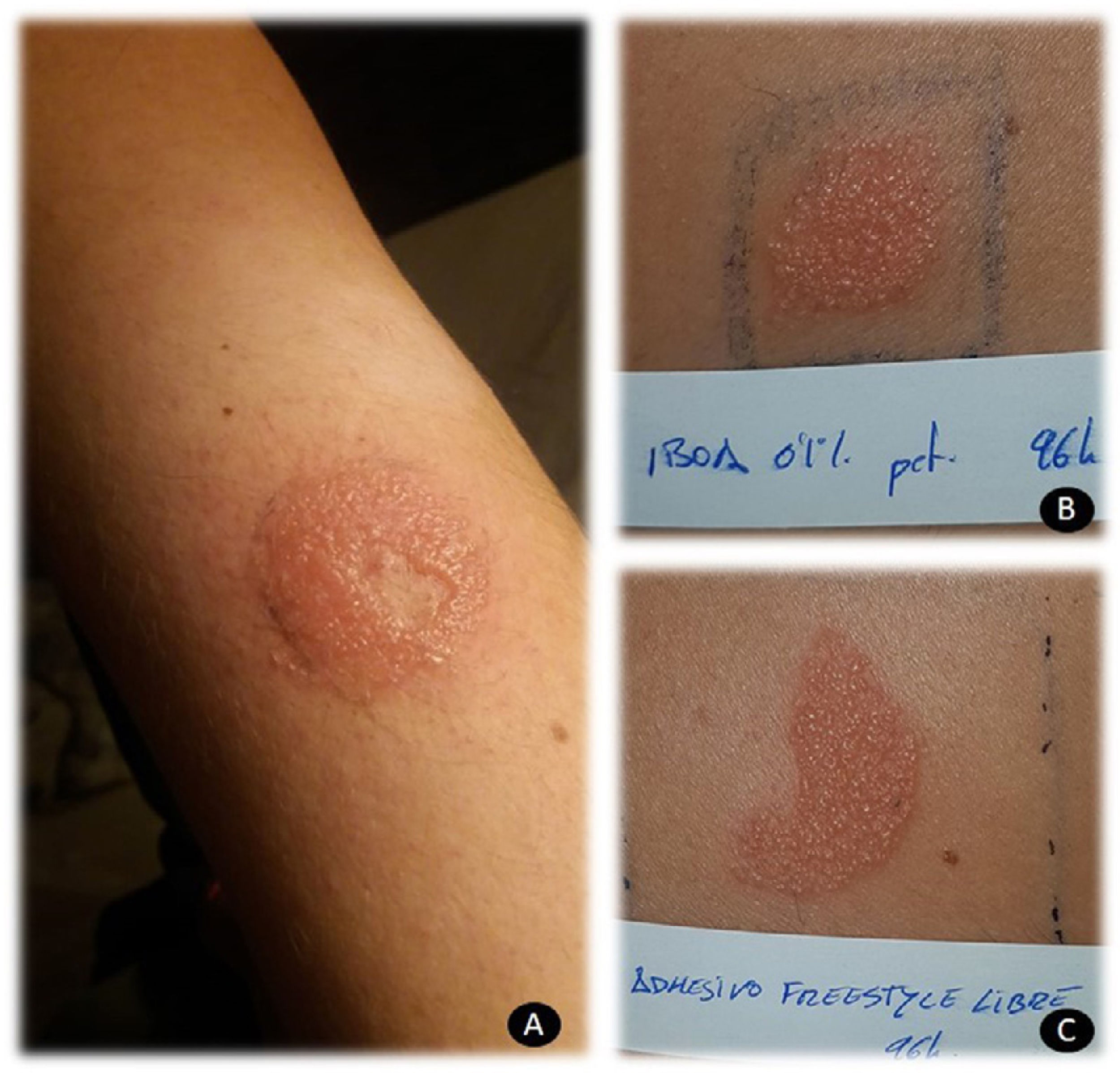
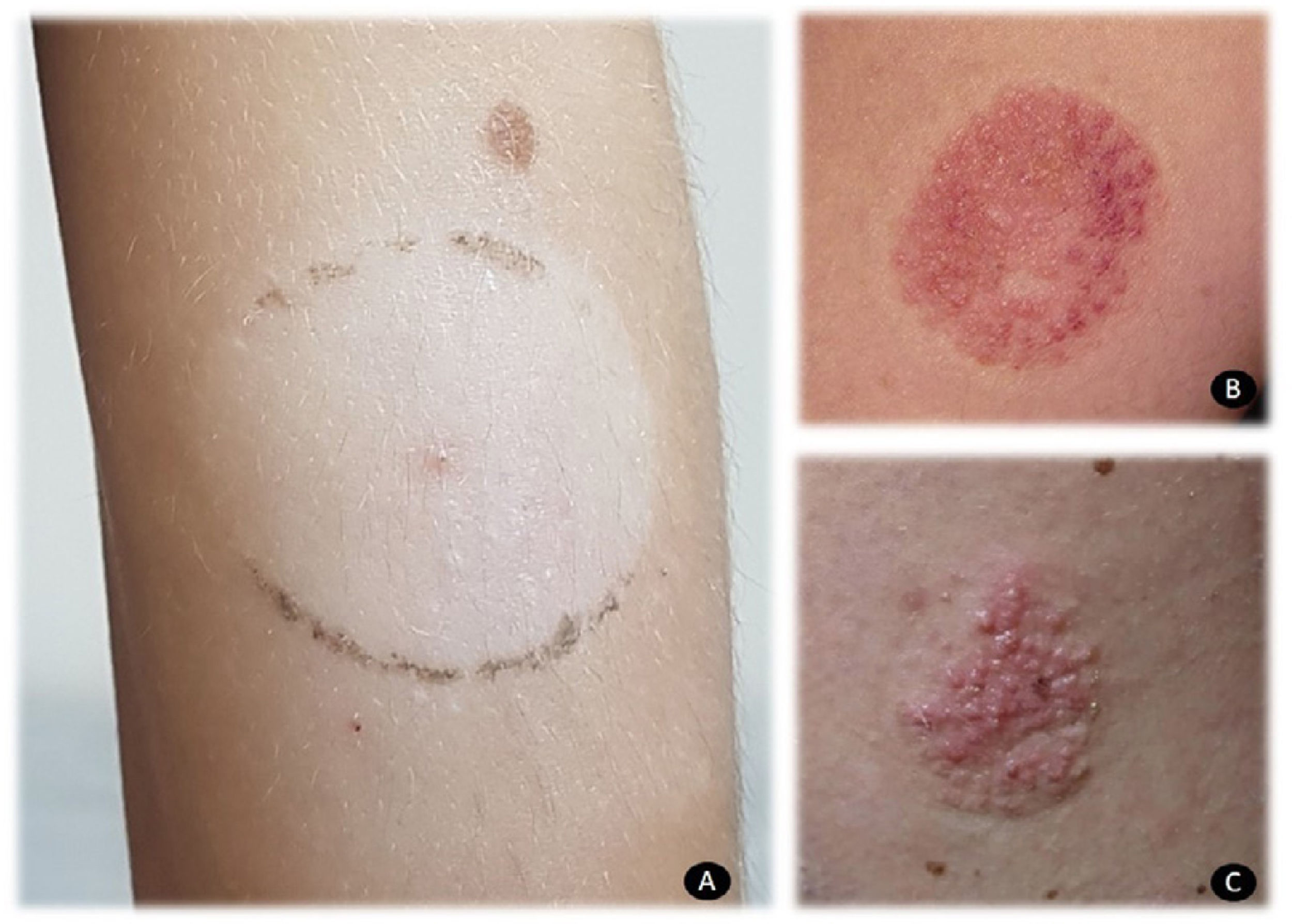
Patch testing is crucial for confirming allergic contact dermatitis in patients who use these devices. Adhesive as is, in addition to testing IBOA 0.1% pet (allergen marketed by Chemotechnique Diagnostics), makes it possible to correctly identify patients with true allergic contact dermatitis. Local reactions are usually vesicular and severe (Fig. 1). It is also important to take into account that IBOA does not seem to cross-react with other acrylates. In my department, all patients were assessed using the acrylate series and reacted only to IBOA. In clinical terms, contact dermatitis to glucose sensors usually manifests as acquired leukoderma (Fig. 2A), which has received attention in the literature,14 reactions with purpuric areas (Fig. 2B), and papulovesicular reactions (Fig. 2C).
The development of new devices for control of glycemia is essential if we are to improve the quality of life of patients with diabetes mellitus. Also key for the management of this condition are adhesives with less sensitizing potency in order to prevent allergic contact dermatitis. In this sense, I believe that avoiding long occlusions (e.g., 14 days for FreeStyle Libre) could play a major role in preventing sensitization, although this has not been demonstrated.
The future seems promising, given that new noninvasive devices could replace current glucose sensors. These include GlucoWise (Medical Expo), which is applied on the finger and can detect glucose levels by means of 65-Hz radiofrequencies. Senseonics Eversense (Senseonics, Inc) is a subcutaneous invasive device that lasts 90 days and will be yet another option in the future. Both models could represent a solution for patients with allergic contact dermatitis caused by currently available glucose sensors. However, in real terms, the high price of the devices, which will probably not be covered, will limit patients' access to these modern glucose sensors.
I think that, for now, given the gradual increase in the frequency of cases, all dermatology reference centers treating patients with contact dermatitis, including those associated with medical devices used in diabetes mellitus, should have access to the most common allergens, namely, IBOA, Abitol, the specific cyanoacrylate series, and, in the best case scenario, N,N-dimethylacrylamide (which can be purchased from Sigma-Aldrich), even though it must be prepared in a laboratory or hospital pharmacy. A meticulous study of patients with suspected contact dermatitis should enable us to detect the culprit allergens; therefore, it is necessary to have access to those mentioned here. In special situations, cooperation with the endocrinology and pediatrics departments can enable us to draw up a detailed clinical history and provide the patient with the option of requesting a change of sensor (to one that is not funded), without having to pay for it.
Please cite this article as: Navarro-Triviño FJ. Reacciones cutáneas a sensores de glucosa: presente y futuro. Actas Dermosifiliogr. 2021;112:389–391.