We thank Dr. Pizarro for the opportunity to discuss the usefulness of sentinel lymph node biopsy (SLNB) in the treatment of cutaneous melanoma. In his letter he makes several points. The first is that lymphadenectomy can be curative if the affected nodes are dissected.1 We agree with this view: the survival curves to which he refers are not a description of the natural history of the disease (without intervention) but rather refer to patients who were followed up and in whom affected lymph nodes were excised when regional disease was detected. In this scenario, the alternative to SLNB is not inaction but rather observation and excision of affected nodes when metastasis is detected clinically or by ultrasound.
We also agree that SLNB has no effect on patient survival. In a huge study undertaken to determine whether SLNB increased survival—the Multicenter Selective Lymphadenectomy Trial (MSTL-1)2—1661 patients were randomized, starting in 1994, and were followed up for 10 years. This was such a remarkable effort that it is extremely unlikely to be repeated. The results of MSTL-1 provide a clear and definitive answer to the question posed above. The answer is that melanoma specific survival does not increase in patients who have undergone SLNB. And this is clear despite the optimistic biases that affected the reporting of MSTL-1, such as the authors' decision to ignore the study’s primary outcome (the description of overall survival) to the extent that an unsuccessful request has been made for the raw data be made public so that it can be reanalyzed.3 If undertaking SLNB has no beneficial effect on survival, why choose to undergo a surgical intervention that is expensive, invasive, and associated with the risk of morbidity?
Dr. Pizarro’s argument is that, hypothetically, SLNB is a simpler procedure than delayed lymphadenectomy and that, in patients with a positive SLNB, it achieves local control with fewer complications and sequelae. This hypothesis, which must be verified empirically, is both attractive and debatable.3 The main secondary objective of the MSTL-1 protocol was the description of complications. But this data was also, suspiciously, omitted from the final report of the study. In any case, this possible regional control in a few patients is obtained at the expense of performing an intervention with general anesthesia and possible complications,4 from which at least 72% of patients will derive no benefit. To obtain a better understanding of the relative risks of the two options, it would, once again, be important for MSTL-1 data to be released.
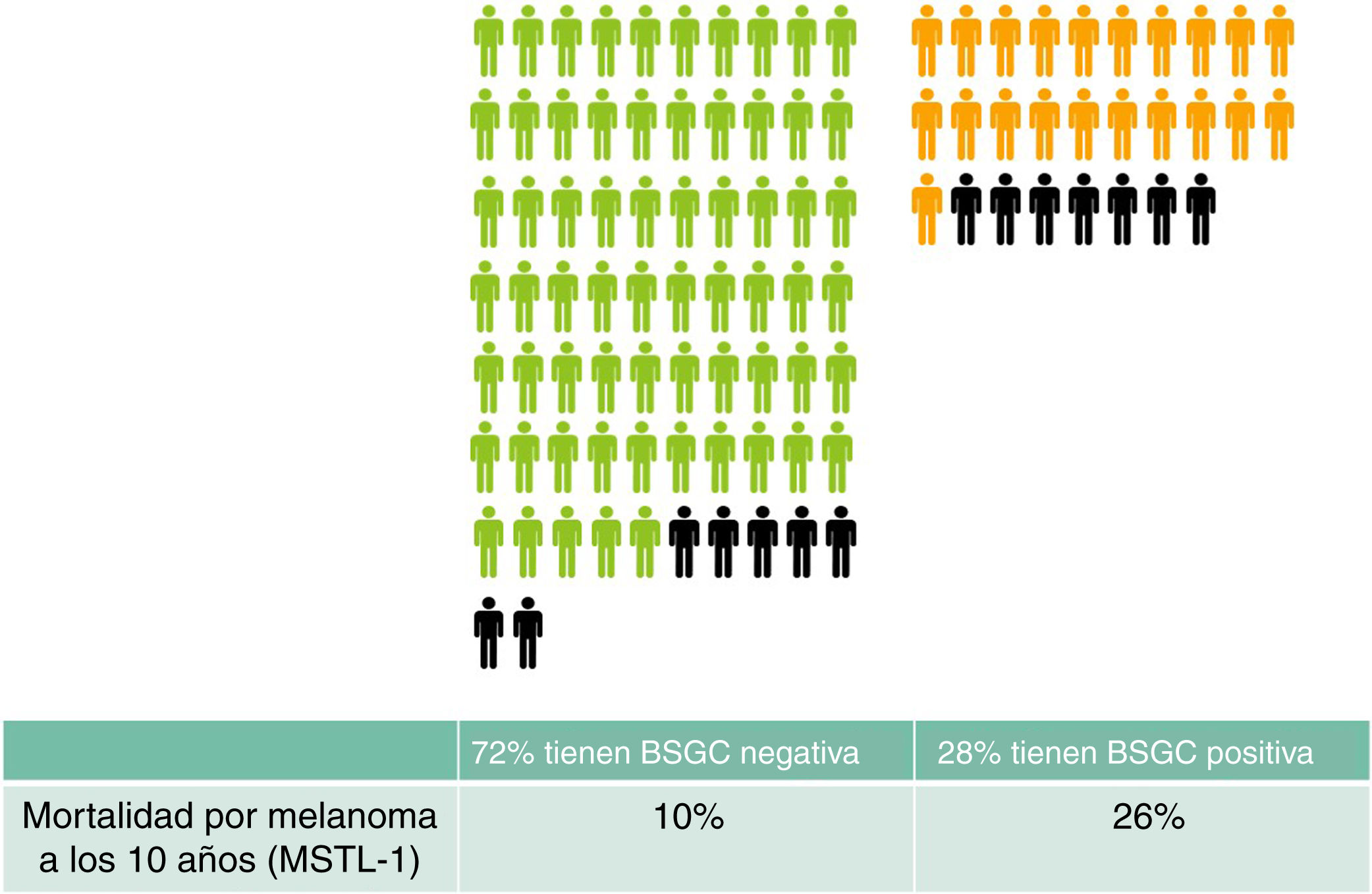
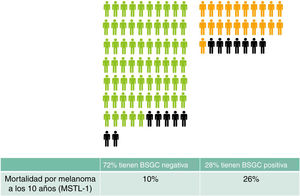
Finally, with respect the remaining half-truth about the sentinel node, namely that “SLNB can facilitate the selection of patients who may benefit from adjuvant treatment”, a few simple calculations will give us a clearer idea. In a group of 100 people with a similar profile to the participants of MSTL-1 (Breslow > 1 and Clark > III or Clark > IV) SLNB will, approximately, be positive in 28 and negative in 72 patients.4 According to the MSTL-1 results, patients with a positive SNLB will have a 10 year melanoma-specific mortality of 26% versus 10% in patients with a negative SLNB.2 The people who may die from the spread of their melanoma would appear to be the potential candidates for adjuvant treatment. What would happen to the group of 100 people after 10 years? In total, 14 of the 100 would have died from their melanoma: 7 of those would have had a positive SLNB (28 × 0.26) and 7 a negative SLNB (72 × 0.10). By offering adjuvant immunotherapy only to those with positive SLNB we would avoid the use of indiscriminate treatment but we would deprive half of those who could have benefited from treatment (Fig. 1). Can this be considered a good selection method?
Expected outcomes in 100 patients with a profile similar to that of participants of the MSTL-1 trial (Breslow > 1 and Clark > III or Clark > IV). Patients with negative (green) SLNB and positive SLNB (orange) have been grouped separately. Patients who will die of melanoma after 10 years are indicated in black. Presumably, these are the patients who would benefit most from adjuvant immunotherapy.
We believe that it is not a good selection method and that the situation could get even worse if, despite the ethical doubts that arise,5 clinical immunotherapy trials requiring a prior SLNB continue to be carried out. This practice could create a paradoxical situation, because the performance of SLNB would become a necessary condition for receiving immunotherapy. Probably, the decision to undertake SLNB would be made less restrictive in order to benefit more patients. This would, in turn, bring the percentage of positive results to less than the current 28% and, in absolute terms (as can be seen by recalculating the data presented in Fig. 1), there would be more patients who would benefit from immunotherapy in the group with a negative SLNB! A method is needed to identify patients who would benefit from adjuvant immunotherapy, but it must be a method that can provide greater reliability than that offered by earlier noninvasive methods, and SLNB does not offer this level of reliability. The literature tells us that the use of data such as Breslow thickness and ulceration yield similar predictive results.6,7 To be in a position to explain the reality to our patients, we would like to see the results in Fig. 1 using other selection methods.
We believe that SLNB is an example of "medical reversal": an intuitively attractive treatment that is widely adopted before there is empirical evidence of its effectiveness and which we then cling to, even when the evidence demonstrates that it does not offer any benefits and may even be harmful.8 In the case of SLNB, the evidence shows that the intervention produces morbidity, that it does not improve survival, and that it provides little or no information that can identify patients who would benefit from adjuvant treatment.
Please cite this article as: García-Doval I, Espinosa-Pereiro C, Zulaica Gárate A. Biopsia selectiva del ganglio centinela en melanoma: ni utilidad terapéutica, ni es buena para seleccionar los pacientes que podrían beneficiarse de la inmunoterapia adyuvante. Actas Dermosifiliogr. 2020;111:537–539.