The variant of cutaneous squamous cell carcinoma, known as high-risk cutaneous squamous cell carcinoma, has a higher incidence of metastasis. Certain characteristics define this high-risk tumor and are predictors of increased risk of metastasis, although the risk factors are not yet well established. This observational retrospective study of 392 cases of high-risk cutaneous squamous cell carcinoma of the head and neck explored the tumor risk factors for metastasis and the association between metastasis and death. The only factor with a significant positive association with mortality was tumor invasion of noncutaneous structures. A total of 6.6% of the tumors metastasized, and mortality was 30.8%. These findings are consistent with observations reported in the literature.
Existe una variante del carcinoma epidermoide cutáneo, denominada de alto riesgo, que se caracteriza por tener una mayor incidencia de metástasis. Algunas características del tumor definen esta variante de alto riesgo, como factores predictores de metástasis, pero todavía no se encuentran bien establecidas. Este es un estudio observacional retrospectivo en el que incluimos 392 casos de carcinoma epidermoide cutáneo de cabeza y cuello. Realizamos un análisis de los factores de riesgo tumorales para el desarrollo de metástasis y la relación entre la presencia de metástasis y la mortalidad del paciente. Únicamente encontramos una relación positiva y significativa entre la invasión tumoral de estructuras extradérmicas y el desarrollo de metástasis. El 6,6% de los tumores desarrollaron metástasis, con una mortalidad del 30,8%. Estos datos se encuentran en concordancia con los resultados publicados en estudios previos.
Cutaneous squamous cell carcinoma of the skin (cSCC) is the second most common nonmelanoma skin cancer.1 It affects the head and neck in between 75% and 90% of cases and has the greatest metastatic potential of all skin cancers.2
The metastatic rate of cSCC of the head and neck varies according to the series, but there is what is known as a high-risk variant that metastasizes more frequently than others.1–3 This variant is characterized by a series of clinical and histological criteria associated with higher mortality and in particular significantly reduced 5-year survival.1–3 Cervical metastases are diagnosed by imaging studies (usually a computed tomography [CT] scan), the results of which determine both the need for adjuvant therapy and prognosis. Adjuvant (regional) therapy consists of cervical lymph node dissection and, depending on the results of the histologic examination, radiation therapy.
The aim of this study was to investigate the behavior of cSCC of the head and neck according to the presence of high-risk factors in patients treated in a plastic surgery department.
Material and MethodsWe performed an observational, retrospective study of all patients with cSCC of the head and neck who underwent surgical excision in the plastic surgery department of Hospital Universitario Miguel Servet in Zaragoza, Spain between 2006 and 2011.
The inclusion criteria were treatment with surgical excision and histologic confirmation of invasive carcinoma. Patients without confirmation of metastasis after cervical lymph node dissection were excluded due to the impossibility of determining subsequent development of metastasis with certainty.
The patients’ medical records were reviewed to collect information on the following variables: demographic characteristics; death; cause of death (regional, metastatic cSCC of the head and neck, other); presence of high- versus low-risk tumor characteristics, namely size (≥5cm [high-risk] vs. <5cm [low-risk]), degree of tumor differentiation (Broders classification III [high-risk] vs. I or II [low-risk]), surgical margins (clear vs. affected), tumor location (upper or lower lip and pinna [high-risk] vs. other [low- risk]), invasion of deep extradermal structures (yes vs. no); regional treatment (yes vs. no); diagnostic imaging result (positive, negative, imaging study not performed); histologic confirmation of cancer spread in lymph node dissection after imaging study (metastasis vs. no metastasis); and development of metastasis (yes vs. no). The tumors were staged using the sixth (before 2010) and seventh (after 2010) editions of the American Joint Committee on Cancer (AJCC) staging system for cSCC. As this was a retrospective study, we had to unify and adapt the different factors defined as high-risk in our series to the information available in the patients’ records.
We analyzed associations between high-risk factors assessed both individually and as a whole (total number of factors) and risk of metastasis. Patients who underwent lymph node dissection and did not develop metastasis were excluded from this analysis. We also studied the link between metastasis and death.
The significance of differences between categorical variables was analyzed using the χ2 test and contingency tables. Logistic regression and the Wald test were used to investigate associations between tumor characteristics and the development of metastasis. Statistical significance was set at a P value of .05.
All statistical analyses were carried out in SPSS, version 16.0.
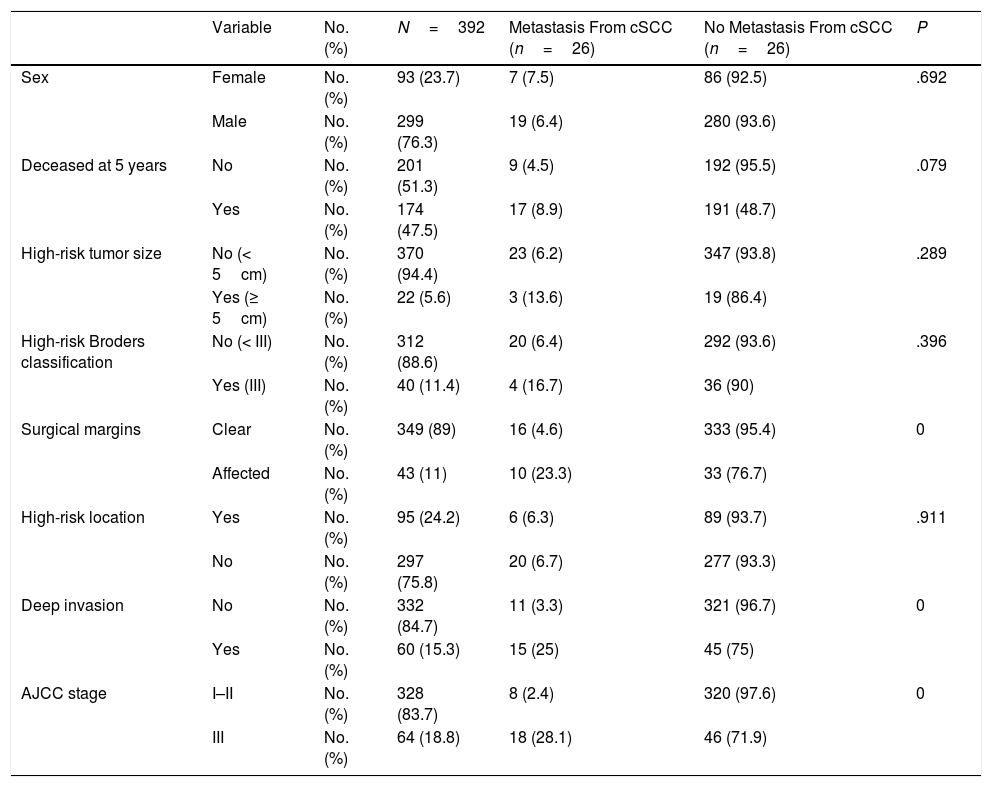
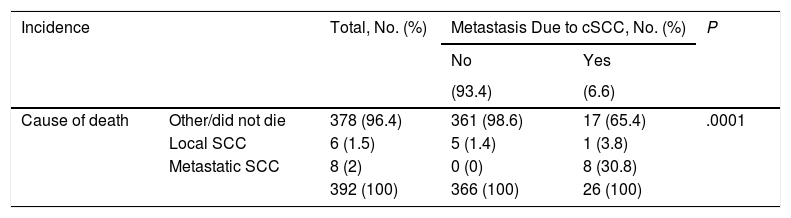
ResultsWe studied 392 patients with cSCC of the head and neck. Twenty-six developed metastasis during follow-up, which was at least 5 years in all cases. The general characteristics of the sample are summarized in Table 1. Affected surgical margins, invasion of extradermal structures (deep invasion), and stage III cSCC (stage T4 or N1 according to the sixth AJCC classification and stage T3 or N1 according to the seventh AJCC classification) were associated with a higher incidence of metastasis (P<.05). The distribution and characteristics of patients who developed metastasis are shown in Table 2.
General characteristics of Patients With Cutaneous Squamous Cell Carcinoma (cSCC) of the Head and Neck.
| Variable | No. (%) | N=392 | Metastasis From cSCC (n=26) | No Metastasis From cSCC (n=26) | P | |
|---|---|---|---|---|---|---|
| Sex | Female | No. (%) | 93 (23.7) | 7 (7.5) | 86 (92.5) | .692 |
| Male | No. (%) | 299 (76.3) | 19 (6.4) | 280 (93.6) | ||
| Deceased at 5 years | No | No. (%) | 201 (51.3) | 9 (4.5) | 192 (95.5) | .079 |
| Yes | No. (%) | 174 (47.5) | 17 (8.9) | 191 (48.7) | ||
| High-risk tumor size | No (< 5cm) | No. (%) | 370 (94.4) | 23 (6.2) | 347 (93.8) | .289 |
| Yes (≥ 5cm) | No. (%) | 22 (5.6) | 3 (13.6) | 19 (86.4) | ||
| High-risk Broders classification | No (< III) | No. (%) | 312 (88.6) | 20 (6.4) | 292 (93.6) | .396 |
| Yes (III) | No. (%) | 40 (11.4) | 4 (16.7) | 36 (90) | ||
| Surgical margins | Clear | No. (%) | 349 (89) | 16 (4.6) | 333 (95.4) | 0 |
| Affected | No. (%) | 43 (11) | 10 (23.3) | 33 (76.7) | ||
| High-risk location | Yes | No. (%) | 95 (24.2) | 6 (6.3) | 89 (93.7) | .911 |
| No | No. (%) | 297 (75.8) | 20 (6.7) | 277 (93.3) | ||
| Deep invasion | No | No. (%) | 332 (84.7) | 11 (3.3) | 321 (96.7) | 0 |
| Yes | No. (%) | 60 (15.3) | 15 (25) | 45 (75) | ||
| AJCC stage | I–II | No. (%) | 328 (83.7) | 8 (2.4) | 320 (97.6) | 0 |
| III | No. (%) | 64 (18.8) | 18 (28.1) | 46 (71.9) |
Abbreviations: AJCC, American Joint Committee on Cancer.
Patients With Metastasis From Cutaneous Squamous Cell Carcinoma (cSCC) of the Head and Neck: Incidence and Mortality.
| Incidence | Total, No. (%) | Metastasis Due to cSCC, No. (%) | P | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| (93.4) | (6.6) | ||||
| Cause of death | Other/did not die | 378 (96.4) | 361 (98.6) | 17 (65.4) | .0001 |
| Local SCC | 6 (1.5) | 5 (1.4) | 1 (3.8) | ||
| Metastatic SCC | 8 (2) | 0 (0) | 8 (30.8) | ||
| 392 (100) | 366 (100) | 26 (100) | |||
In the logistic regression analysis investigating predictors of metastasis (tumor size, Broder classification, surgical margins, location, deep invasion, number of high risk factors), the only significant predictor according to the Wald test was deep invasion (P=.0001, odds ratio [OR], 10.32). This factor was also significantly associated with tumor size (<5cm vs. ≥5cm) (P=.004).
DiscussioncSCC of the head and neck is characterized by the presence of certain risk factors associated with an increased risk of subclinical metastasis in the regional lymph nodes.4 These high-risk factors, however, have not yet been well established5–7 and numerous studies have attempted to identify criteria that could be used to develop a prognostic model for this setting.
One review published in 2014 identified just 6 independent risk factors for cSCC: histologic differentiation, anatomic location, thickness, diameter, perineural and lymphovascular invasion, and immunosuppression.2 We explored 4 of these variables in our series. Because of the retrospective nature of our study, the patients’ records contained insufficient data to analyze perineural and lymphovascular invasion and immune status. Nonetheless, neither immune status nor lymphovascular invasion is included as a high-risk factor in the latest version of the AJCC classification.8
We also analyzed surgical margins and total number of high-risk factors. Surgical margin status has been linked to patient survival.9 The protocol at our hospital requires excision until clear margins are obtained in all cases. In our series, positive margins and deep invasion were associated with a higher incidence of metastasis. Neither of these variables, however, can be considered independent predictors of metastasis4 and numerous authors agree that risk is defined by a combination of factors.1,2,5,7,10–12 We would like to highlight, however, the importance of complete excision with negative margins in cSCC of the head and neck.
The only factor that retained its significance as an independent predictor of metastasis in the multivariate analysis was invasion of deep structures (P=.0001, OR, 10.32); 25% of patients with deep invasion developed metastasis compared with just 3.3% of those without. This finding coincides with reports by authors who have performed similar analyses to predict metastasis in cSCC of the head and neck4,10; it is also consistent with the finding by Martorell et al.1 that tumor thickness is the strongest independent predictor of metastasis in this setting.1 Deep invasion in our series was defined as tumor invasion of extradermal structures. A tumor size of over 5cm was also directly associated with deep invasion (P=.003).
Twenty-two of the tumors in our series were larger than 5cm. The prevalence of cSCC of the head and neck in our series is high compared with most other reports in the literature. We believe that this high frequency can be explained by the geographic distribution and characteristics of the population served by our hospital, which are linked to delayed diagnosis and referral to the plastic surgery department. Tumors measuring 5cm or more were associated with a higher rate of metastasis than smaller tumors (13.6% vs. 6.2%, P=.172).
Most studies published to date have described a tumor diameter greater than 2cm1,7,11–13 and a Breslow thickness of 4mm4,5,11,14 as risk factors for metastasis. In our series, a tumor size of 5cm or more was significantly associated with metastasis, but this does not mean that we do not agree with the results reported by other studies. We used depth of invasion to determine deep growth, as the retrospectively reviewed histopathologic reports included information on Clark level not Breslow thickness.
The patients in our series were followed for at least 5 years, which is when 96% of metastases occur.2 The incidence of metastasis from cSCC of the head and neck in the literature varies considerably, with rates ranging from 0.1% to 47.3%.2,3,5,11,13
In our series, 6.6% of patients developed metastasis. This rate is based on the total number of tumors, that is, we did not classify tumors by level of risk, as the criteria for doing this have not yet been well established. Differences in published rates can be attributed to the use of different factors to define risk, and it is also likely that the risk of metastasis increases with years.2,13
Patients who develop metastasis are at an increased risk of death. Survival rates, like metastatic rates, also vary from one series to the next. In general, the 5-year survival rate for untreated metastatic cSCC is less than 35%.2,3,5 In our series, 30.8% of patients died due to metastatic cSCC (Table 2). In the overall sample, however, disease-specific death was due to local tumor invasion (constitutional syndrome, acute hemorrhage) in 7 patients (one of whom already had regional metastasis) and the development of metastasis in 8. This distinction is poorly documented in the literature, and even though metastasis is a cause of death in cSCC, a significant proportion of patients die due to local invasion. In our series, most of the deaths (96.4%) were due to a cause other than cSCC and only 8 of the 26 patients who developed metastasis died as a result. Our results should thus be interpreted with caution, as we do not know if patients who died of a cause other than cSCC might have developed metastasis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bernal Martínez ÁJ, Fernández Letamendi N, Delgado Martínez J, Sampietro de Luis JM, Gómez-Escolar Larrañaga L, Sanz Aranda E. Factores de riesgo y mortalidad del carcinoma epidermoide cutáneo de cabeza y cuello. Actas Dermosifiliogr. 2020;111:325–328.