Biological therapies have changed the management and treatment of several immune-mediated disorders, including psoriasis. These therapies include tumor necrosis factor inhibitors, and monoclonal antibodies targeting the IL-23/IL-17 axis, known for its role in the disease pathogenesis.1
Risankizumab is a selective IL-23 inhibitor with a proven high efficacy and a favorable safety profile.2 However, its use in severely immunocompromised patients is limited, as these patients are often excluded from clinical trials.
We report 2 case reports of patients with severe plaque psoriasis who were immunosuppressed due to solid organ transplantation. In both patients risankizumab therapy was started due to its efficacy and safety profile.
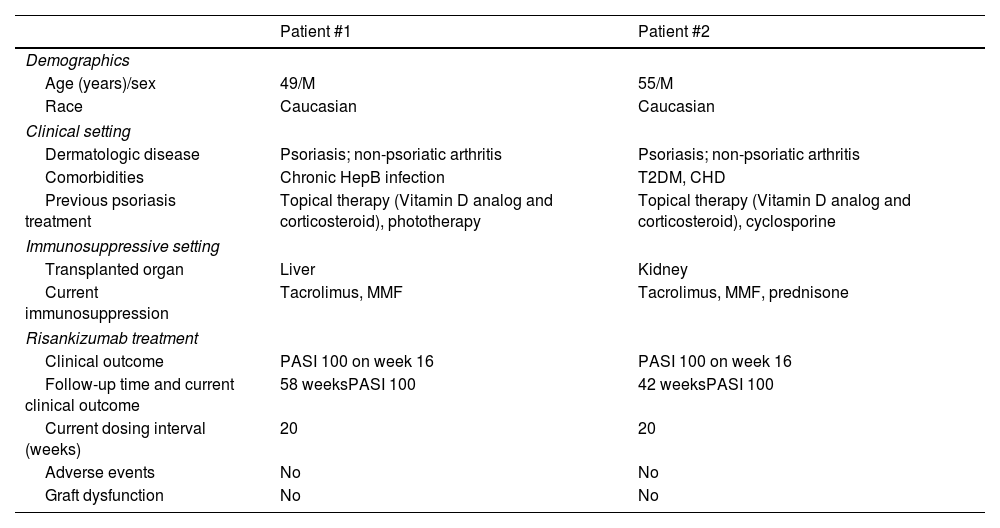
Case report #1 is a 49-year-old man with untreated chronic hepatitis B (HepB) infection who developed severe toxic hepatitis after treatment for latent pulmonary tuberculosis with isoniazid, which eventually led to liver transplantation. Immunosuppressive therapy with tacrolimus and mycophenolate mofetil (MMF) was initiated, along with suppressive therapy for HepB infection. The patient was referred to our center due to severe psoriasis, with a baseline Psoriasis Area Severity Index (PASI) of 14. Risankizumab was started.
Complete skin clearance (PASI100) was achieved on week 16 without any adverse events being reported. Due to sustained clinical remission, the intervals between the different administrations of risankizumab were spaced out 20 weeks. Currently, on week 58, the patient remains in clinical remission and no adverse events or increased HepB viral load have been reported.
Case report #2 is a 55-year-old man with plaque psoriasis, type 2 diabetes, coronary heart disease, and progressive chronic kidney disease. In 2014, the patient underwent renal transplantation due to end-stage renal disease and began immunosuppressive therapy with tacrolimus, MMF, and prednisone. One year after transplantation there was a noticeable flare-up of his psoriasis, baseline PASI of 14, and the patient was eventually referred to our center. A course of cyclosporine was attempted, but since no significant clinical improvement was observed risankizumab was initiated.
On week 16, PASI100 was achieved with no recorded adverse reactions, or an impaired renal function. Currently, on week 42, and due to the sustained clinical remission, the patient is on risankizumab every 20 weeks and continues to be monitored on a routine basis.
Further clinical data is shown in Table 1.
Clinical characteristics of solid organ transplant recipients with severe plaque psoriasis on risankizumab.
| Patient #1 | Patient #2 | |
|---|---|---|
| Demographics | ||
| Age (years)/sex | 49/M | 55/M |
| Race | Caucasian | Caucasian |
| Clinical setting | ||
| Dermatologic disease | Psoriasis; non-psoriatic arthritis | Psoriasis; non-psoriatic arthritis |
| Comorbidities | Chronic HepB infection | T2DM, CHD |
| Previous psoriasis treatment | Topical therapy (Vitamin D analog and corticosteroid), phototherapy | Topical therapy (Vitamin D analog and corticosteroid), cyclosporine |
| Immunosuppressive setting | ||
| Transplanted organ | Liver | Kidney |
| Current immunosuppression | Tacrolimus, MMF | Tacrolimus, MMF, prednisone |
| Risankizumab treatment | ||
| Clinical outcome | PASI 100 on week 16 | PASI 100 on week 16 |
| Follow-up time and current clinical outcome | 58 weeksPASI 100 | 42 weeksPASI 100 |
| Current dosing interval (weeks) | 20 | 20 |
| Adverse events | No | No |
| Graft dysfunction | No | No |
CHD, coronary heart disease; HepB, hepatitis B; M, male; MMF, mycophenolate mofetil; PASI, Psoriasis Area and Severity Index; T2DM, type 2 diabetes mellitus.
IL-23 plays a crucial role in sustaining chronic inflammation through activation and differentiation of Th17 cells, which in turn, produce pro-inflammatory cytokines such as IL-17. Risankizumab selectively targets IL-23, by binding to the p19 subunit, thus interrupting the inflammatory process.3
The immunosuppressive potential of biological therapies has long raised safety concerns, limiting their use in cases of increased risk of infection and malignancy, such as immunocompromised patients due to solid organ transplantation. However, evidence has confirmed the favorable safety profile of biological agents selectively targeting IL-23. This is likely attributed to the IL-23 regulatory function rather than a suppressive role.1
In fact, risankizumab has not shown an increased risk of severe opportunistic infection development. For example, risankizumab appears to be safe in patients with latent tuberculosis (TB) infection, with no evidence suggesting an increased risk of disease reactivation, even in patients naïve to TB prophylaxis.4 Additionally, a recent retrospective trial that assessed the safety profile of risankizumab in patients with chronic viral hepatitis confirmed the absence of a reduced liver function or elevated viral loads; instead, treated patients experienced a reduced progression of liver fibrosis. This positive outcome is likely attributed to the involvement of IL-23 in this pathological process.5
Also, the risk of malignancies does not seem to increase with the long-term use of IL-23 inhibitors.6 Preclinical studies have shown that IL-23 levels correlate with a poor prognosis in many human neoplasms. Due to the observed tumor-promoting effects of IL-23 it has been suggested that the inhibition of this cytokine, and its downstream pathways, may have protective effects vs tumorigenesis and metastasis.7
Finally, the possibility of increasing the interval between risankizumab doses may be relevant for complex patients such as the ones reported earlier. In the phase III clinical trial IMMhance, a significant durability of response following risankizumab withdrawal was seen, and a similar finding was noted with guselkumab, another IL-23 inhibitor.8 Additionally, the GUIDE study is currently evaluating the possibility of increasing the dose interval in patients on guselkumab, and preliminary data have shown the feasibility of extending it to every 16 weeks in a subgroup of patients.9 Recently it was shown that administering risankizumab “as needed”, once clinical remission is achieved, could be a feasible maintenance scheme for patients with psoriasis. This clinical phenomenon is likely related to the effects of IL-23 inhibition on tissue resident memory cells and T-regulatory cells.10
In conclusion, due to its mechanism of action, efficacy, and safety profile, risankizumab can be considered a safe option for complex patients with psoriasis and concomitant immunosuppression, such as solid organ transplant recipients.
Conflict of interestsM.L. and A.M.L. have no conflicts of interest. T.T. declares the following conflicts of interest: AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius Kabi, Janssen, LEO Pharma, Eli Lilly, MSD, Mylan, Novartis, Pfizer, Samsung-Bioepis, Sanofi-Genzyme, Sandoz, and UCB.