Oral minoxidil (2,4-diamino-6-piperidinopyrimidine-3-oxide) is a potent peripheral vasodilator that was initially used in the 1970s for the treatment of severe refractory hypertension at doses of 10 mg/day to 100 mg/day.1 Owing to the development of hypertrichosis in a quarter of the patients who received this drug, a topical formulation was developed to treat pattern hair loss, first in men and subsequently in women.
The mechanism of action of minoxidil on the hair follicle is poorly understood. Through the action of its active form, minoxidil sulfate, topical minoxidil shortens the telogen phase and causes resting follicles to prematurely enter the anagen phase. It also prolongs the anagen phase and increases hair follicle size.2 Oral administration reduces blood pressure by inducing relaxation of vascular smooth muscle through the opening of sarcolemmal ATP2-dependent potassium channels. Minoxidil's effect on the hair follicle is also thought to be mediated by minoxidil sulfate-induced opening of potassium channels, although this remains to be demonstrated (Fig. 1).
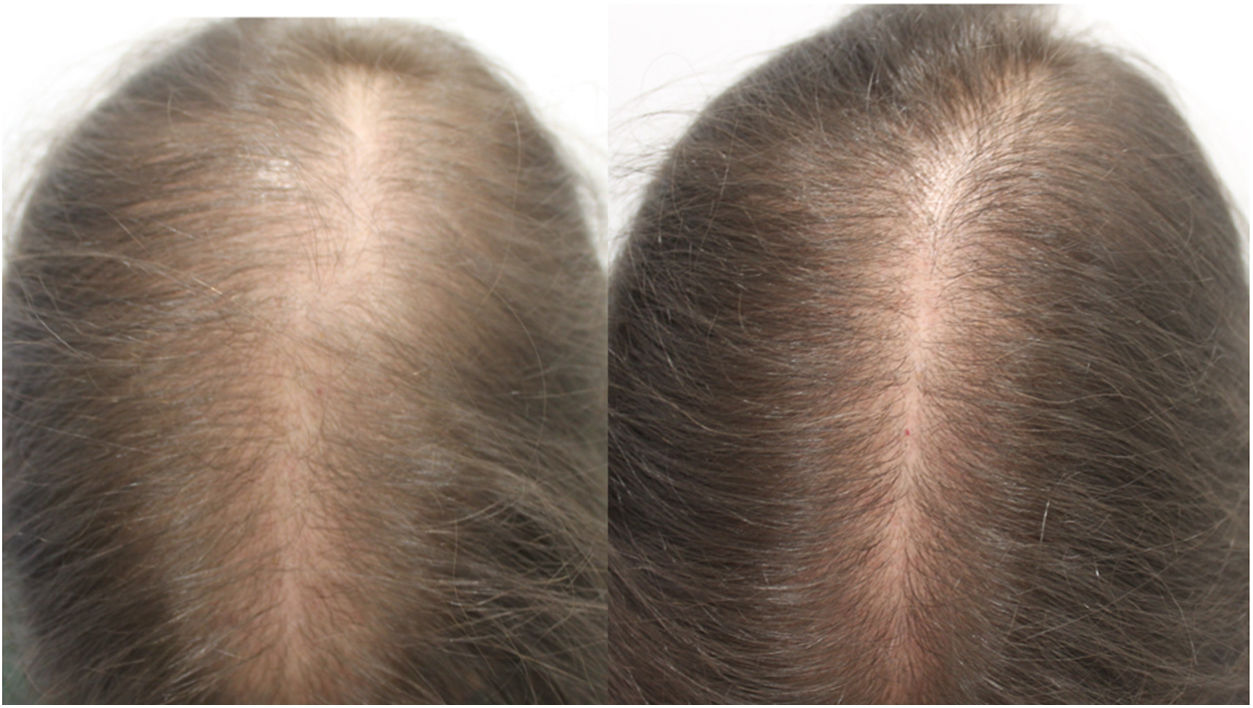
Due to the adverse effects observed at standard doses, oral minoxidil is not widely used for the treatment of alopecia. Evidence indicates that oral minoxidil at low doses (0.5–2.5 mg/d) is beneficial in the treatment of isolated cases of permanent alopecia caused by chemotherapy,2 chronic telogen effluvium,3 female pattern hair loss,4 and monilethrix.5 In our experience, oral minoxidil is a particularly interesting option for the treatment of female pattern hair loss and chronic telogen effluvium (1 mg/d) and male pattern hair loss (5 mg/d). It can be administered as monotherapy or in combination with a low-intensity topical minoxidil regimen (e.g. 3 days per week).
The main adverse effect is facial hypertrichosis, the risk of which is dose-dependent (4% risk at 0.25 mg/d, 38% risk at 1 mg/d).4,5 This adverse effect is reversible upon discontinuation of treatment, and in our experience is generally mild and does not pose a significant problem for the patient. Isolated cases (<2% of patients) of postural hypotension and tachycardia have also been reported,4 and therefore measurement of baseline blood pressure is recommended. In patients with blood pressure values <90/60 mmHg and those with postural hypotension sodium chloride (50 mg) should be added to the formulation.4 In our professional experience doses of 1 mg/day are safe and rarely cause hypotension.
In Spain, oral minoxidil is sold as 10-mg tablets for the treatment of severe arterial hypertension, but it can be formulated in capsules. In patients with female pattern hair loss the addition of spironolactone (25 mg) reduces the risk of edema in the lower limbs and potentiates the therapeutic effects of minoxidil, although theoretically spironolactone can worsen postural hypotension.4
In conclusion, oral minoxidil at low doses is an interesting therapeutic alternative in patients who decline or do not tolerate topical administration, especially in cases of female pattern hair loss.
Please cite this article as: Pindado-Ortega C, Saceda-Corralo D, Vañó-Galván S. FR – Minoxidil oral para el tratamiento de la alopecia androgénica femenina y otras alopecias. Actas Dermosifiliogr. 2019;110:861–862.