Vitiligo is a depigmenting skin disease with a global prevalence of 1%. While multiple therapeutic options exist, none of them is completely satisfactory, especially in universal vitiligo, where depigmentation of the residual areas of pigment using chemical methods, such as hydroquinone monobenzyl ether (HMBE), may be cosmetically acceptable, as it produces complete, almost irreversible depigmentation. Other depigmenting substances exist, however, such as phenol, have been described previously but with little clinical evidence.
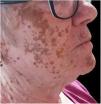
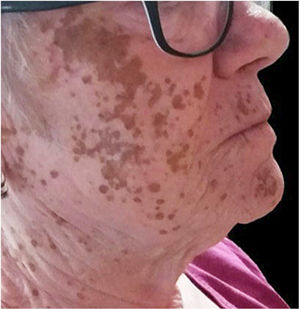
A 67-year-old woman with vitiligo that had appeared in childhood and was universally distributed since 14 years earlier visited our department seeking alternative depigmenting therapeutic options, as she presented areas of residual pigmentation on the face, chest, and shoulders, which had not responded to prior treatment with HMBE (Fig. 1).
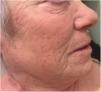
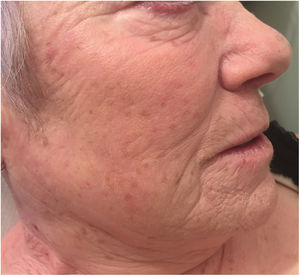
An 88% aqueous solution of phenol was applied selectively, starting on the pigmented areas on the right cheek, as they were small areas that minimized systemic absorption, toxicity, and adverse effects. This was achieved by evaluating the response and tolerance of the patients and by applying cold compresses to alleviate pain immediately after frosting was observed. Given the good response, 4 applications were performed on the same section, achieving complete depigmentation, and the same procedure was performed on the other pigmented areas with excellent results and no evidence of repigmentation until 1 year after the procedure; the patient, who complied strictly with photoprotection, was very satisfied (Fig. 2).
The melanotoxic effects of phenol have been previously documented, but its depigmenting mechanism of action appears to be complex. The common characteristic of these products is their chemical structure, which includes a phenol group made up of a benzene ring with a hydroxyl side chain, a structure that is shared with the amino acid tyrosine. Potent phenols act as tyrosine analogs, interfering with the melanogenesis pathway, and initial hypotheses suggested that depigmenting chemicals that entered the melanogenesis pathway generated toxic metabolites that destroy the melanocytes. Tyrosine and other enzymes of melanogenesis bind covalently to phenols, as does tyrosine, generating reactive oxygen species and activating the response of the proteins released, autophagy, and exosomes, which supply adjacent immune cells with new antigens, initiate an inflammatory response, and activate autoreactive T cells, thus initiating an autoimmune response that results in their destruction.1–3
They also induce glutathione depletion, which may increase the immunogenicity of melanosomal proteins. The pigmented cells exposed to phenol activate specific T cells, also reacting against other melanocytes not directly stressed by exposure. This would explain depigmentation at a distance from these compounds through a mechanism analogous to that which occurs in contact sensitization.4
The use of phenol has not been described previously in guidelines on the management of vitiligo, even though its use may be safe and economically viable. In Brazil, however, it has been introduced successfully in clinical practice at a concentration of 88%, as a depigmenting therapy for universal vitiligo; nevertheless, a case reported in Iran obtained no response to single-drug therapy with phenol and treatment was supplemented with cryotherapy.5,6
Phenol is an aromatic hydrocarbon derived from coal tar and is used as a chemical peeling agent. Its effect varies depending on the concentration and the surface area to which it is applied. Concentrations above 80% produce denaturalization and rapid and irreversible coagulation of epidermal proteins, resulting in the formation of a barrier that prevents the chemical from penetrating the deep dermis, whereas when diluted to 50%, it acts as a keratolytic agent and disrupts the sulfur bridges, thus increasing its penetration beyond the dermis and causing greater destruction and systemic absorption.7
Complications may include scarring, dyschromia, and eczema herpeticum. High doses are toxic, and it must not, therefore, be applied over large areas, given that it has a marked corrosive action, either due to ingestion, inhalation, or direct contact. Cellular uptake is rapid and passive due to its lipophilic nature and signs of systemic toxicity appear shortly after exposure. Target organs are the liver, kidneys, lungs, and cardiovascular system. When used by qualified experts, however, it does not usually cause complications. Repigmentation of the skin may occur if patients do not protect themselves adequately from the sun.8
Although the only treatment currently approved by the US Food and Drugs Administration (FDA) for vitiligo is HMBE, few published cases describing the efficacy of phenol and isolated studies demonstrating its mechanism of action exist. Our patient presented a satisfactory response to the selective application of 88% phenol, with no complications and no relapse; we therefore consider it to be an excellent depigmenting therapeutic option in universal vitiligo with areas of pigmentation that do not respond to HMBE.
FundingThis study has not received funding of any kind.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Alomar A, Marrón Hernández M, Bittencourt F. Islotes de pigmentación residual en paciente con vitiligo universal, tratados con peeling de fenol al 88%. Actas Dermosifiliogr. 2021;112:284–285.