Upadacitinib is an oral Janus kinase inhibitor (JAKi) approved to treat moderate-to-severe atopic dermatitis (AD) of patients aged ≥12 years. Its efficacy and safety profile vs placebo has already been demonstrated in clinical trials1 and real-life data is starting to be reported in the scientific medical literature.
We conducted a retrospective cohort study collecting data from adolescents and adults with severe refractory atopic dermatitis treated with daily upadacitinib 30mg from May 2022 through April 2023 at the Dermatology Unit of Hospital Universitario Ramon y Cajal, Madrid, Spain. Effectiveness was measured using the validated Investigator Global Assessment for Atopic Dermatitis (IGA-AD) scale (scores of 0 or 1 with ≥2-grade reduction compared to baseline), and patients reaching Eczema Area and Severity Index (EASI) 75 on weeks 4, 12 and 24. All treatment-related adverse events (AE) were collected.
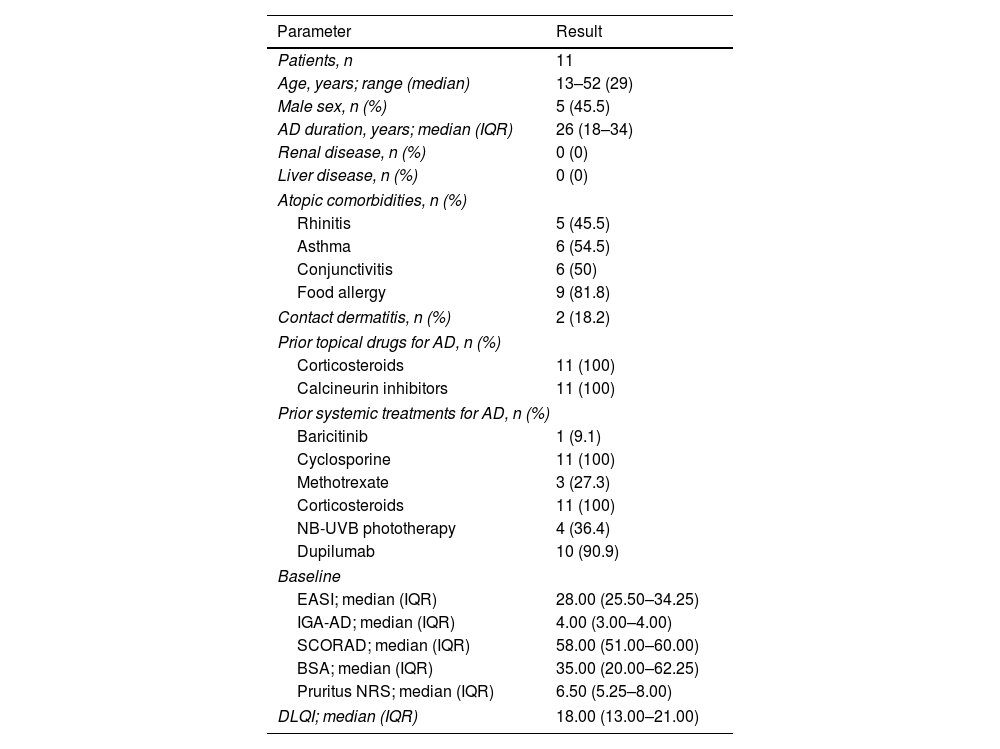
Eleven patients were included, with ages ranging from 13 to 52 years (median, 29). All had been treated unsatisfactorily with topical and oral corticosteroids and cyclosporine, 10/11 (90.9%) were on dupilumab; and 1/11 (9.1%) on baricitinib, without achieving acceptable disease control. The baseline characteristics are shown in Table 1.
Baseline clinical and demographic characteristics of patients with severe atopic dermatitis on upadacitinib.
| Parameter | Result |
|---|---|
| Patients, n | 11 |
| Age, years; range (median) | 13–52 (29) |
| Male sex, n (%) | 5 (45.5) |
| AD duration, years; median (IQR) | 26 (18–34) |
| Renal disease, n (%) | 0 (0) |
| Liver disease, n (%) | 0 (0) |
| Atopic comorbidities, n (%) | |
| Rhinitis | 5 (45.5) |
| Asthma | 6 (54.5) |
| Conjunctivitis | 6 (50) |
| Food allergy | 9 (81.8) |
| Contact dermatitis, n (%) | 2 (18.2) |
| Prior topical drugs for AD, n (%) | |
| Corticosteroids | 11 (100) |
| Calcineurin inhibitors | 11 (100) |
| Prior systemic treatments for AD, n (%) | |
| Baricitinib | 1 (9.1) |
| Cyclosporine | 11 (100) |
| Methotrexate | 3 (27.3) |
| Corticosteroids | 11 (100) |
| NB-UVB phototherapy | 4 (36.4) |
| Dupilumab | 10 (90.9) |
| Baseline | |
| EASI; median (IQR) | 28.00 (25.50–34.25) |
| IGA-AD; median (IQR) | 4.00 (3.00–4.00) |
| SCORAD; median (IQR) | 58.00 (51.00–60.00) |
| BSA; median (IQR) | 35.00 (20.00–62.25) |
| Pruritus NRS; median (IQR) | 6.50 (5.25–8.00) |
| DLQI; median (IQR) | 18.00 (13.00–21.00) |
AD, atopic dermatitis; NB-UVB, narrowband ultraviolet B; EASI, Eczema Area and Severity Index; IGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis; SCORAD, SCORing Atopic Dermatitis; NRS, numerical rating scale; BSA, body surface area; DLQI, Dermatology Life Quality Index; IQR, interquartile range.
Regarding effectiveness, all patients reported rapid pruritus relief, which in 72.7% of the patients was absolute; in these patients the median+interquartile range (IQR) time to control was 4.0 days (2.5–5). At baseline, the median+IQR values for EASI and IGA-AD were 28.00 (25.50–34.25) and 4.00 (3.00–4.00), respectively; the SCORAD index, 5.00 (51.00–60.00); and the body surface area (BSA) affected value, 35.00 (20.00–62.25). Regarding patient-reported outcome measures, the median+IQR values for peak pruritus numerical rating scale (NRS) and DLQI were 6.50 (5.25–8.00) and 18.00 (13.00–21.00), respectively.
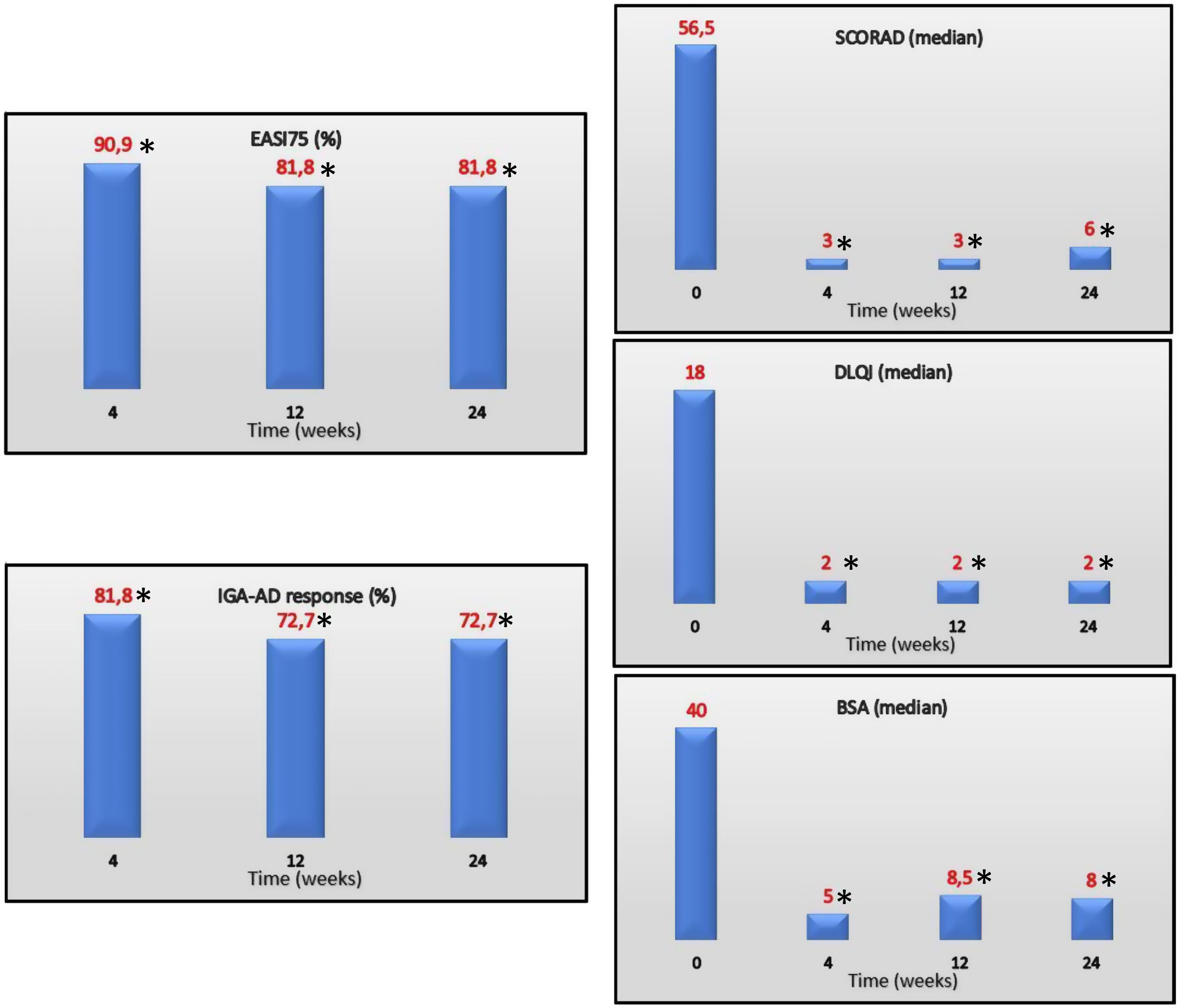
EASI75 (≥75% reduction from baseline EASI) was reached by 90.9%, 81.8% and 81.8% of the patients on weeks 4, 12 and 24, respectively. IGA-AD scores of 0–1 were reached by 81.8%, 72.7% and 72.7% of the patients on weeks 4, 12 and 24, respectively. Median values of DLQI at the follow up were 4, 2, and 2 on weeks 4, 12 and 24, respectively. Efficacy of treatment at the follow up is shown in Fig. 1.
EASI75, IGA-AD 0/1 (%) and SCORAD, DLQI, and BSA (median) over time. EASI, Eczema Area and Severity Index; IGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis of 0 or 1; SCORAD, SCORing Atopic Dermatitis; DLQI, Dermatology Life Quality Index; BSA, body surface area.
The Mann–Whitney U-test was used, when necessary, to calculate statistical differences.
*P≤.001 for differences vs baseline.
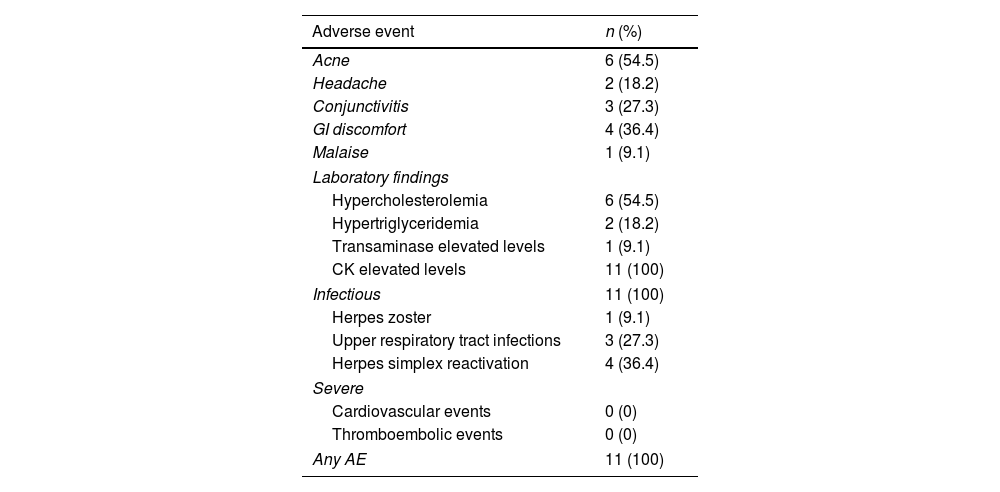
Regarding AE, all patients presented with, at least, 1 mild AE. Analytically, all patients presented with slight elevations of creatine kinase levels at some time at the follow-up; other findings were hypercholesterolemia (54.5%), hypertriglyceridemia (18.2%) and mild elevation of hepatic transaminases (9.1%). Regarding infectious disorders, 1/12 (9.1%) experienced an episode of multimetameric herpes zoster. No severe clinical or analytical AE were recorded. All the AE reported are shown in Table 2.
Safety data in patients with severe atopic dermatitis on upadacitinib.
| Adverse event | n (%) |
|---|---|
| Acne | 6 (54.5) |
| Headache | 2 (18.2) |
| Conjunctivitis | 3 (27.3) |
| GI discomfort | 4 (36.4) |
| Malaise | 1 (9.1) |
| Laboratory findings | |
| Hypercholesterolemia | 6 (54.5) |
| Hypertriglyceridemia | 2 (18.2) |
| Transaminase elevated levels | 1 (9.1) |
| CK elevated levels | 11 (100) |
| Infectious | 11 (100) |
| Herpes zoster | 1 (9.1) |
| Upper respiratory tract infections | 3 (27.3) |
| Herpes simplex reactivation | 4 (36.4) |
| Severe | |
| Cardiovascular events | 0 (0) |
| Thromboembolic events | 0 (0) |
| Any AE | 11 (100) |
AE, adverse effect; CK, creatine kinase.
Measure UP 1 and 2 clinical trials achieved EASI75 in 76.3% of the patients on week 16 at the follow-up, while we saw a slightly higher EASI75 value on weeks 12 and 24 (81.8% in both).1 In former studies on the real clinical practice with larger samples, Pereyra-Rodriguez et al. reported EASI75 and IGA-AD responses on week 16 of 76.7% and 62.8%, respectively (sample of 43 patients), which is lower compared to our findings.2 On the other hand, Chiricozzi et al. reported higher EASI75 outcomes on week 16 (97.5%) in a sample of 43 patients.3 Focusing on studies that include only patients with refractory AD as we do (ineffectiveness of dupilumab or JAKi), Feraru et al. found an IGA-AD response similar to that of our study at the 24-week follow-up in 12 adults (75%), while De Greef et al. reported a somewhat lower IGA-AD response (71.4%) at the long-term follow-up.4,5 In studies with shorter follow-ups, Dal Bello et al. achieved an EASI75 outcome in all 10 patients with refractory AD included in the study at the 8-week follow-up, while Napolitano et al. reported an EASI75 of 75% (16 patients included) at the 16-week follow-up.6,7
We should mention the excellent control of pruritus achieved by upadacitinib in our series (72.7% of the patients vs 60% reported in clinical trials), and the short time until the patient becomes itch-free (median, 4 days), a variable not considered in former studies that may be of interest since itching represents the main AD-related symptom and has a direct impact on the patients’ quality of life.
In conclusion, our efficacy findings are consistent with those reported both in previous clinical trials and real clinical practice reports. In our experience, upadacitinib is a reliable option when patient circumstances demand prompt itching relief.
The strength of our study is that it was a real-life experience of patients with AD refractory to multiple treatments. The main limitations of the study include its small sample size and retrospective data collection.
Conflict of interestThe authors declare that they have no conflict of interest.