The blue rubber bleb nevus syndrome (BRBNS) or Bean syndrome is a relatively rare congenital disorder characterized by the presence of multiple cutaneous and visceral venous malformations, particularly in the digestive tract; the lesions progressively increase in number and size.1 These patients, particularly in the severe cases, typically develop intense chronic iron deficiency anemia secondary to recurrent gastrointestinal bleeding, and repeated transfusions and long-term iron therapy are necessary. There is currently no curative treatment. Surgical treatment of the lesions by endoscopic argon-plasma fulguration2 or intestinal resection with end-to-end anastomosis3 may be required in serious cases. Treatment with antiangiogenic drugs, such as corticosteroids, propranolol, and interferon alfa, has also been described, but the results have been poor. Recently, the efficacy of oral sirolimus (also known as rapamycin) has been reported in 2 cases of BRBNS.4,5
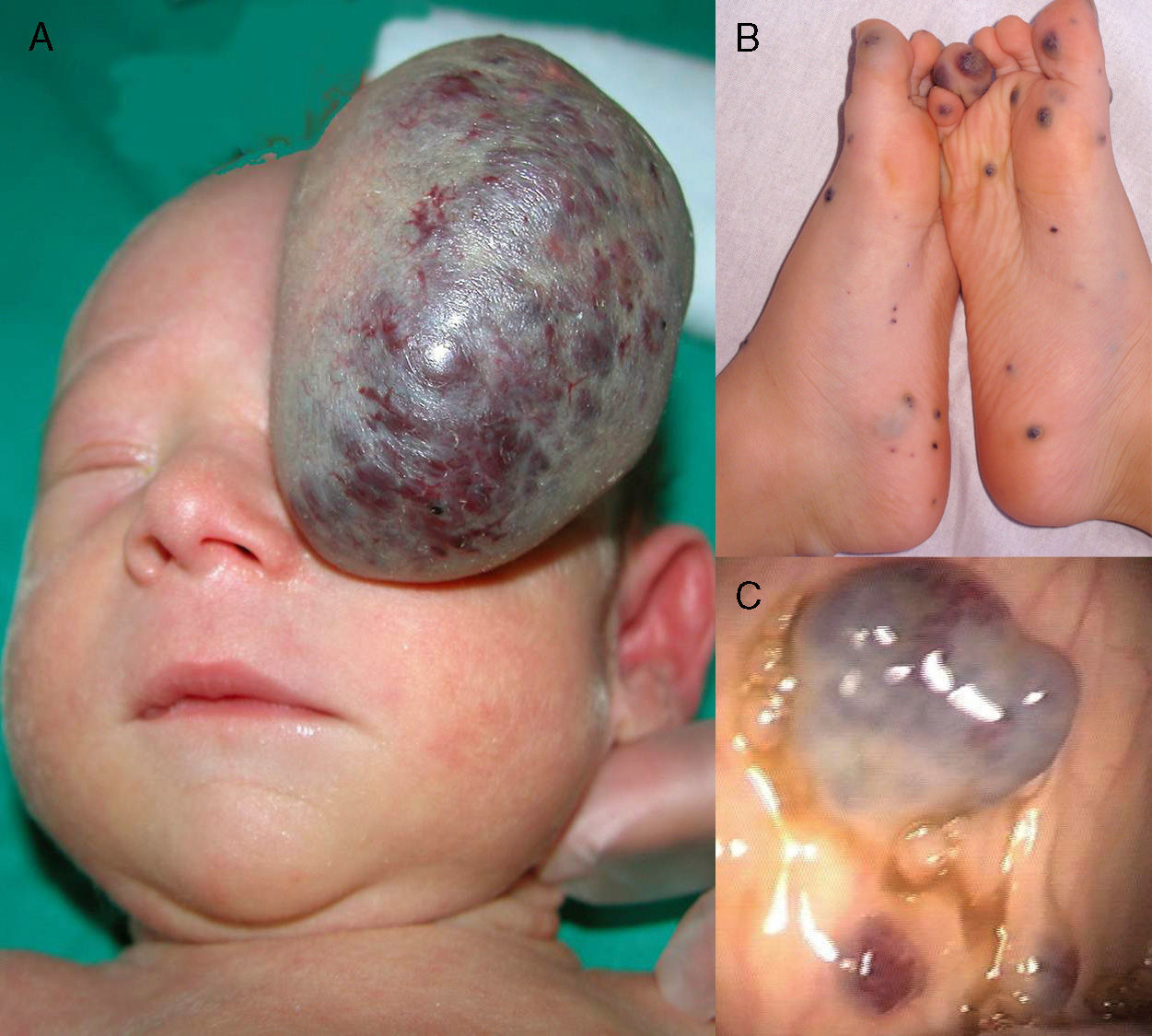
We present the case of a full-term newborn infant with a congenital tumor of 7cm diameter that arose from the fronto-orbital region and occluded the left eye (Figure 1A). The lesion was surgically removed at 5 days of life. Histology revealed the presence of dilated vessels with a thin endothelium, calcifications, and a granulomatous reaction. The medical history excluded problems during gestation or delivery, as well as any family history of vascular disorders. Histological study of the placenta was not performed.
A, A large, pedunculated vascular tumor with superficial telangiectasias. The tumor had a blue-purpuric color centrally and was pale peripherally, similar to a rapidly involuting congenital hemangioma. B, Multiple bluish skin lesions on the feet, typical of blue rubber bleb nevus syndrome. C, Venous malformations in the gastrointestinal tract.
At 4 months, a soft, bluish subcutaneous tumor appeared in the area of the scar, associated with multiple bluish papules of 0.2 to 0.5cm diameter arising mainly on the trunk and limbs. With a suspected diagnosis of BRBNS, gastrointestinal endoscopy was performed and revealed multiple vascular malformations along the length of the intestine. Over the following years, the lesions in the skin (Figure 1B) and intestine (Figure 1C) progressively increased in number and size, affecting nerves and joints, causing pain and interfering with walking; the patient also presented recurrent episodes of severe gastrointestinal bleeding. Secondary to the intestinal bleeding, the patient developed severe iron deficiency anemia that required repeated transfusions and oral and intravenous iron therapy. Some of the symptomatic skin lesions were treated by excision or sclerosis. At 6 years of age, laser ablation of the intestinal malformations was performed, achieving a partial response but with subsequent recurrence. In view of the patient's poor clinical course and the persistence of the anemia, requiring intravenous iron therapy and more than 3 transfusions in the last year, when the patient was 8 years of age it was decided to commence treatment with oral sirolimus at a starting dose of 0.05mg/kg. The dose rapidly had to be reduced to half (0.025mg/kg) due to the presence of elevated sirolimus levels in the blood; the dose reduction achieved levels in the appropriate range. Tolerance to treatment was good and no adverse effects were observed. The skin lesions showed a slight improvement, and the hemoglobin levels normalized within a month; no further blood transfusions were necessary after 12 months of continuous treatment.
Sirolimus is an immunosuppressant drug that acts by inhibiting the mTOR (mammalian target of rapamycin) pathway. Its main use up to now has been as an oral medication to prevent rejection in kidney transplant. Given its anticancer properties through its inhibition of neoangiogenesis and of tumor cell proliferation, it has been found to be useful to reduce the number and size of tumors in patients with tuberous sclerosis6–8 as well as in certain serious vascular abnormalities.9,10 The first description of the efficacy of sirolimus at a dose of 0.05-0.1mg/kg to reduce the size of the skin lesions and decrease gastrointestinal bleeding in a patient with BRBNS was published in April 2012. More recently, in November 2013, sirolimus was used successfully at a dose of 4mg to resolve chronic anemia in an 18-year-old patient with BRBNS.
In our case, a lower dose of sirolimus (0.025mg/kg) has been effective, leading to the cessation of gastrointestinal hemorrhage, normalization of the hemoglobin levels, and no further need for transfusion.
In the future, study of the expression of proteins of the mTOR pathway in the skin lesions may enable us to predict the response to drugs that block their activation, such as sirolimus, temsirolimus, everolimus, and others.
ConclusionSirolimus appears to be a therapeutic option in blue rubber bleb nevus syndrome. However, further studies are necessary to confirm its efficacy and to establish the dose regimen in this rare condition.
Please cite this article as: Ferrés-Ramis L, Knöpfel N, Salinas-Sanz J.A., Martín-Santiago A. Rapamicina para el tratamiento del síndrome del nevus azul en tetina de goma. Actas Dermosifiliogr. 2015;106:137–138.