Dermatologic surgery is associated with a very low risk of complications. There is no widely accepted, evidence-based protocol with recommendations for postoperative wound care after dermatologic surgery. In this narrative review, we will be discussing the evidence on surgical wound care products and procedures. Overall, we found relatively few studies and, in many cases, a lack of statistically significant differences, possibly because of the low rate of complications. We’ll be discussing the evidence on when we should initiate wound care procedures and their frequency, the type of ointment and antiseptics that should be applied, and the type of dressings that should be used. Despite the very few studies available on postoperative wound care following dermatologic surgery, there is sufficient evidence as to not recommend the use of prophylactic topical antibiotics. We also analyze the currently available evidence on surgical wound care in special situations, such as management of skin grafts, partial skin graft donor sites, xenografts/biomembranes, and surgical wounds to the legs.
La cirugía dermatológica asocia un riesgo de complicaciones muy bajo. No existe un protocolo universalmente aceptado sobre las recomendaciones de curas poscirugía dermatológica. En esta revisión narrativa, discutimos la evidencia sobre productos y procedimientos para el cuidado de la herida quirúrgica. En general, encontramos escasos estudios y en muchas ocasiones, falta de diferencias estadísticamente significativas, posiblemente dada la baja tasa de complicaciones. Discutimos la evidencia sobre cuándo iniciar las curas y su frecuencia, el tipo de ungüento a aplicar, los antisépticos y el tipo de apósito a utilizar. Pese a los escasos estudios sobre las curas tras cirugía dermatológica, existe evidencia suficiente para desaconsejar la utilización de antibióticos tópicos profilácticos. También analizamos la evidencia publicada sobre el cuidado de la herida quirúrgica en situaciones especiales, como son los injertos cutáneos, las zonas dadoras de injertos cutáneos de espesor parcial, los xenoinjertos/biomembranas y las heridas quirúrgicas en las piernas.
Dermatologic surgery has a low rate of complications,1 with surgical wound infection (SWI) rates between 0.7 and 4.0%, even in the absence of prophylactic antibiotics, preoperative skin preparation, or use of sterile gloves.2,3 Additionally, SWIs are generally mild and easy to treat.2
The physiological wound healing process is complex, involving neutrophils, macrophages, fibroblasts, and keratinocytes, and consisting of several phases: inflammatory, proliferative, and remodeling (Table 1). In recent years, some studies have suggested that the skin microbiome may also play an important role in the healing process.4 Post-dermatologic surgery wounds care is a controversial topic, and there is no universally accepted protocol to this date. Numerous studies show wide variability in recommendations, such as antiseptic use, topical antibiotics, dressing type, and frequency of care, among others.5
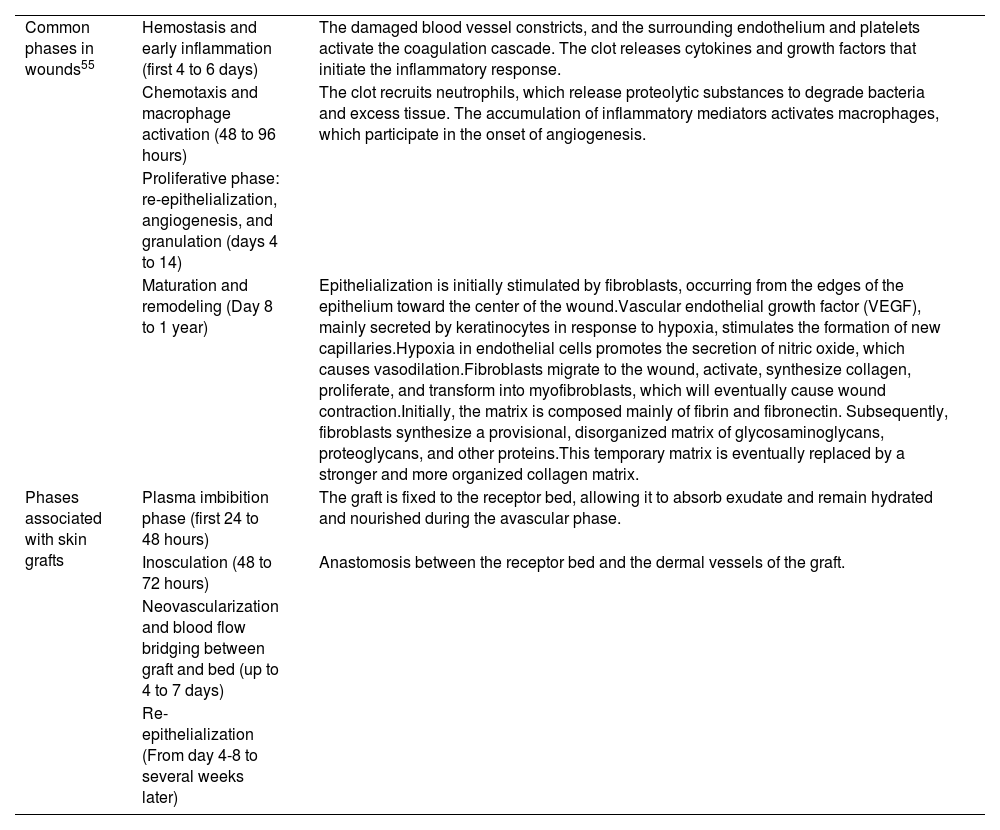
Physiological process of wound healing.
| Common phases in wounds55 | Hemostasis and early inflammation (first 4 to 6 days) | The damaged blood vessel constricts, and the surrounding endothelium and platelets activate the coagulation cascade. The clot releases cytokines and growth factors that initiate the inflammatory response. |
| Chemotaxis and macrophage activation (48 to 96 hours) | The clot recruits neutrophils, which release proteolytic substances to degrade bacteria and excess tissue. The accumulation of inflammatory mediators activates macrophages, which participate in the onset of angiogenesis. | |
| Proliferative phase: re-epithelialization, angiogenesis, and granulation (days 4 to 14) | ||
| Maturation and remodeling (Day 8 to 1 year) | Epithelialization is initially stimulated by fibroblasts, occurring from the edges of the epithelium toward the center of the wound.Vascular endothelial growth factor (VEGF), mainly secreted by keratinocytes in response to hypoxia, stimulates the formation of new capillaries.Hypoxia in endothelial cells promotes the secretion of nitric oxide, which causes vasodilation.Fibroblasts migrate to the wound, activate, synthesize collagen, proliferate, and transform into myofibroblasts, which will eventually cause wound contraction.Initially, the matrix is composed mainly of fibrin and fibronectin. Subsequently, fibroblasts synthesize a provisional, disorganized matrix of glycosaminoglycans, proteoglycans, and other proteins.This temporary matrix is eventually replaced by a stronger and more organized collagen matrix. | |
| Phases associated with skin grafts | Plasma imbibition phase (first 24 to 48 hours) | The graft is fixed to the receptor bed, allowing it to absorb exudate and remain hydrated and nourished during the avascular phase. |
| Inosculation (48 to 72 hours) | Anastomosis between the receptor bed and the dermal vessels of the graft. | |
| Neovascularization and blood flow bridging between graft and bed (up to 4 to 7 days) | ||
| Re-epithelialization (From day 4-8 to several weeks later) |
The aim of this work is to review the procedures and products recommended for post-dermatologic surgery wound care and discuss the evidence supporting them.
MethodsWe conducted a narrative literature review, searching PubMed and Google Scholar in August 2023 using the Spanish and English terms: “dermatologic surgery”; “wound care”; “surgical wound care”; “skin graft”; “direct closure”; “secondary intention closure”; “biomembrane”; “topical antibiotics”; “petrolatum”; “vaseline.” We included prospective and retrospective clinical studies, clinical trials, systematic reviews, and meta-analyses. Articles were selected based on their relevance. Isolated clinical case reports were excluded.
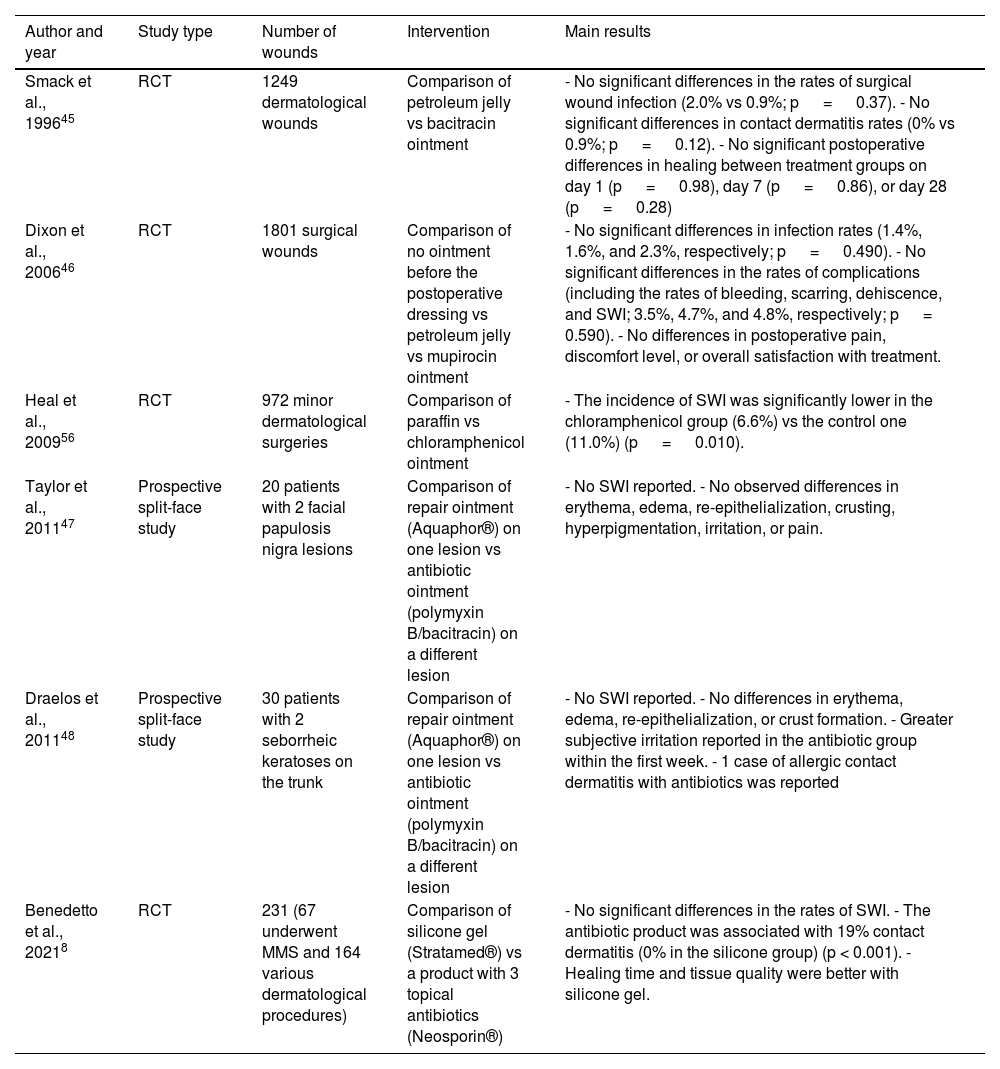
ResultsMoisturizers and topical antibioticsA systematic review and meta-analysis—including 4 randomized clinical trials (RCTs) for a total of 4170 excisions—that evaluated the rates of SWI after dermatologic surgery by comparing topical antibiotics vs vaseline or paraffin found no significant differences.2 A recent meta-analysis found no differences either in the rates of SWI in dermatologic, ophthalmologic, orthopedic, and cardiothoracic surgeries.6 Previously, a Cochrane review7 concluded that the use of topical antibiotics could reduce the risk of SWI vs not using them or using antiseptics. However, it included not only dermatologic procedures but also appendectomies, C-sections, trauma, and cardiothoracic surgeries. A RCT with 231 patients undergoing dermatologic surgery—not included in former systematic reviews—evaluated the application of a topical silicone gel vs an ointment consisting of 3 antibiotics (bacitracin, neomycin, and polymyxin B). A similar incidence of SWI was reported. The risk of contact dermatitis was significantly higher in the topical antibiotic group (19%) vs the silicone one (0%)8 (Table 2).
Prospective studies on the use of topical antibiotics on post-dermatologic procedure wound infection rates.
| Author and year | Study type | Number of wounds | Intervention | Main results |
|---|---|---|---|---|
| Smack et al., 199645 | RCT | 1249 dermatological wounds | Comparison of petroleum jelly vs bacitracin ointment | - No significant differences in the rates of surgical wound infection (2.0% vs 0.9%; p = 0.37). - No significant differences in contact dermatitis rates (0% vs 0.9%; p = 0.12). - No significant postoperative differences in healing between treatment groups on day 1 (p = 0.98), day 7 (p = 0.86), or day 28 (p = 0.28) |
| Dixon et al., 200646 | RCT | 1801 surgical wounds | Comparison of no ointment before the postoperative dressing vs petroleum jelly vs mupirocin ointment | - No significant differences in infection rates (1.4%, 1.6%, and 2.3%, respectively; p = 0.490). - No significant differences in the rates of complications (including the rates of bleeding, scarring, dehiscence, and SWI; 3.5%, 4.7%, and 4.8%, respectively; p = 0.590). - No differences in postoperative pain, discomfort level, or overall satisfaction with treatment. |
| Heal et al., 200956 | RCT | 972 minor dermatological surgeries | Comparison of paraffin vs chloramphenicol ointment | - The incidence of SWI was significantly lower in the chloramphenicol group (6.6%) vs the control one (11.0%) (p = 0.010). |
| Taylor et al., 201147 | Prospective split-face study | 20 patients with 2 facial papulosis nigra lesions | Comparison of repair ointment (Aquaphor®) on one lesion vs antibiotic ointment (polymyxin B/bacitracin) on a different lesion | - No SWI reported. - No observed differences in erythema, edema, re-epithelialization, crusting, hyperpigmentation, irritation, or pain. |
| Draelos et al., 201148 | Prospective split-face study | 30 patients with 2 seborrheic keratoses on the trunk | Comparison of repair ointment (Aquaphor®) on one lesion vs antibiotic ointment (polymyxin B/bacitracin) on a different lesion | - No SWI reported. - No differences in erythema, edema, re-epithelialization, or crust formation. - Greater subjective irritation reported in the antibiotic group within the first week. - 1 case of allergic contact dermatitis with antibiotics was reported |
| Benedetto et al., 20218 | RCT | 231 (67 underwent MMS and 164 various dermatological procedures) | Comparison of silicone gel (Stratamed®) vs a product with 3 topical antibiotics (Neosporin®) | - No significant differences in the rates of SWI. - The antibiotic product was associated with 19% contact dermatitis (0% in the silicone group) (p < 0.001). - Healing time and tissue quality were better with silicone gel. |
RCT, randomized clinical trial; MMS, Mohs micrographic surgery; SWI, surgical would infection.
A prospective study compared the application of vaseline, a reparative ointment (Aquaphor®), and no ointment in 76 patients undergoing Mohs surgery. The study confirmed a higher incidence of crusting in the no-ointment group along with a higher incidence of erythema and inflammation in the reparative ointment group. Both differences were significant.9
Start and frequency of careIt is generally recommended to keep the wound covered for the first 48hours. However, a multicenter RCT (n=857) evaluated the risk of SWI in patients undergoing dermatologic surgery between early dressing removal and bathing with soap and water 12hours after surgery vs late removal after 48hours, finding no significant differences in the rates of SWI.10
A survey of 64 patients undergoing Mohs surgery reported that hydrocolloid dressings applied for an average of 6.4 days without removal were associated with greater comfort, better scar appearance, and higher satisfaction vs conventional daily dressings. Complications such as infections, fever, and pain were similar in both groups.11 Similarly, studies conducted on patients undergoing dermatologic surgery on the legs, where dressings were changed weekly—using compression bandages and zinc oxide—did not show a higher rate of SWI vs conventional daily care.12
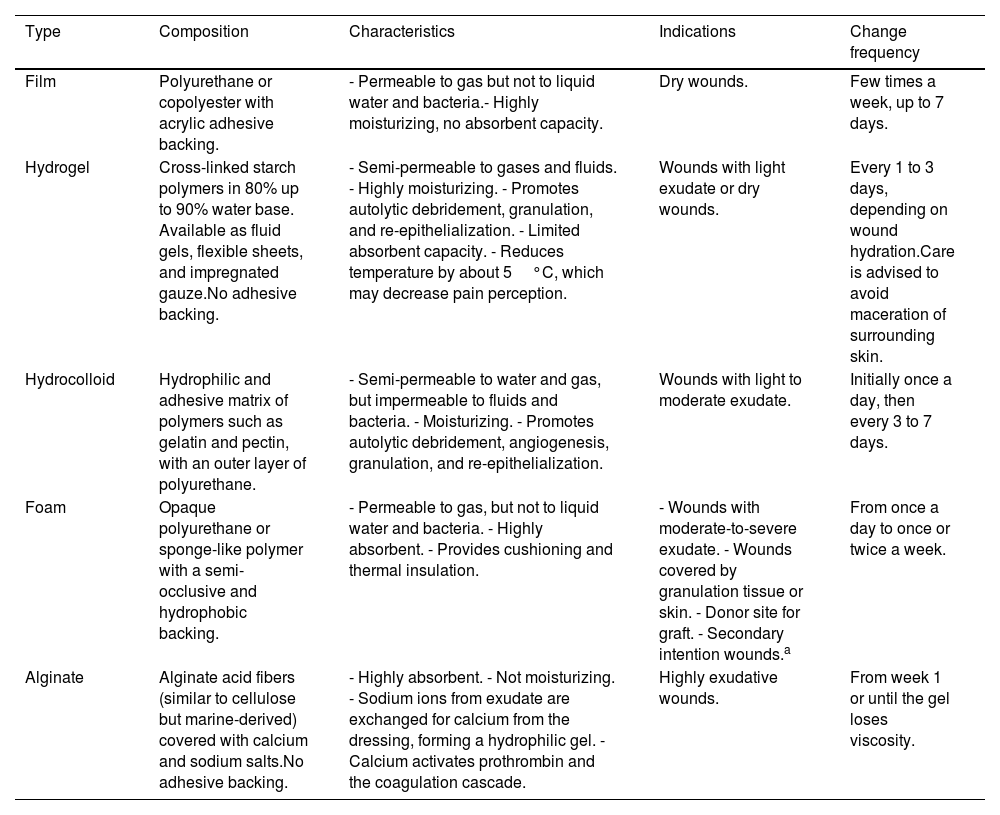
DressingsTraditionally, gauze, bandages, and cotton have been used for postoperative wound coverage. More recently, advanced dressings such as interface films, foams, hydrogels, hydrocolloids, and alginates have been developed (Table 3). Although the frequency of dressing changes depends on the type of dressing, the goal is to space out the care provided.13 A prospective study of 226 small wounds closed by secondary intention, such as shaves and 3mm punch biopsies, showed that the use of advanced occlusive dressings was associated with shorter re-epithelialization time and pain vs traditional dressings.14 Furthermore, as mentioned earlier, the hydrocolloid dressing kept in place for about a week was preferred by the patients over conventional daily care.11
Types of advanced dressings.
| Type | Composition | Characteristics | Indications | Change frequency |
|---|---|---|---|---|
| Film | Polyurethane or copolyester with acrylic adhesive backing. | - Permeable to gas but not to liquid water and bacteria.- Highly moisturizing, no absorbent capacity. | Dry wounds. | Few times a week, up to 7 days. |
| Hydrogel | Cross-linked starch polymers in 80% up to 90% water base. Available as fluid gels, flexible sheets, and impregnated gauze.No adhesive backing. | - Semi-permeable to gases and fluids. - Highly moisturizing. - Promotes autolytic debridement, granulation, and re-epithelialization. - Limited absorbent capacity. - Reduces temperature by about 5°C, which may decrease pain perception. | Wounds with light exudate or dry wounds. | Every 1 to 3 days, depending on wound hydration.Care is advised to avoid maceration of surrounding skin. |
| Hydrocolloid | Hydrophilic and adhesive matrix of polymers such as gelatin and pectin, with an outer layer of polyurethane. | - Semi-permeable to water and gas, but impermeable to fluids and bacteria. - Moisturizing. - Promotes autolytic debridement, angiogenesis, granulation, and re-epithelialization. | Wounds with light to moderate exudate. | Initially once a day, then every 3 to 7 days. |
| Foam | Opaque polyurethane or sponge-like polymer with a semi-occlusive and hydrophobic backing. | - Permeable to gas, but not to liquid water and bacteria. - Highly absorbent. - Provides cushioning and thermal insulation. | - Wounds with moderate-to-severe exudate. - Wounds covered by granulation tissue or skin. - Donor site for graft. - Secondary intention wounds.a | From once a day to once or twice a week. |
| Alginate | Alginate acid fibers (similar to cellulose but marine-derived) covered with calcium and sodium salts.No adhesive backing. | - Highly absorbent. - Not moisturizing. - Sodium ions from exudate are exchanged for calcium from the dressing, forming a hydrophilic gel. - Calcium activates prothrombin and the coagulation cascade. | Highly exudative wounds. | From week 1 or until the gel loses viscosity. |
We did not find any studies comparing the use of antiseptics or detergents such as soap, povidone-iodine, hydrogen peroxide, chlorhexidine, and alcohol solutions, among others, for the management of dermatologic surgical wounds.
For years, it has been suggested that although povidone-iodine may be toxic to fibroblasts and keratinocytes in vitro,15 no healing delays have ever been confirmed.16–19 A systematic review described iodine as superior to other antiseptic agents, such as silver sulfadiazine cream and non-antiseptic dressings in reducing bacterial load and was not associated with impaired healing, or long healing time.20
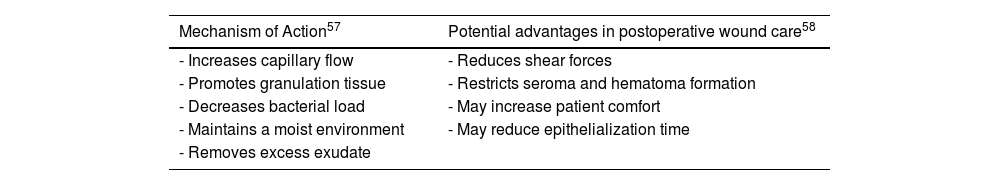
Negative pressure therapyNegative pressure therapy (NPT) may be useful in wound management, offering various advantages (Table 4). In surgical wounds with primary closure, it has not proven particularly significant.21 We only found 1 study in dermatologic surgery: 1 RCT with 49 patients with grafts on their legs after skin cancer excision compared graft success rates with NPT vs dressing and rest. No significant differences were reported between the 2 groups at the 6- and 12-week follow-ups.22 Nonetheless, one meta-analysis with 10 RCTs including 488 patients with partial-thickness skin grafts (PTSGs) for various indications (burns, traumatic wounds, chronic leg wounds, and oncologic excisions in the legs) reported significant differences in viable graft percentage, shorter time to healing (9.18 days vs 12.5 days in the control group), and lower reintervention rates with NPT.23 Similar results were reported in a former meta-analysis of 5 cohort studies and 7 RCTs (653 patients overall).24
Mechanism of action and potential advantages of negative pressure therapy in postoperative wound care.
| Mechanism of Action57 | Potential advantages in postoperative wound care58 |
|---|---|
| - Increases capillary flow | - Reduces shear forces |
| - Promotes granulation tissue | - Restricts seroma and hematoma formation |
| - Decreases bacterial load | - May increase patient comfort |
| - Maintains a moist environment | - May reduce epithelialization time |
| - Removes excess exudate |
For the combination of NPT with xenografts (biomembranes), one RCT with 36 patients with wounds exposing bone or tendon compared the application of the Integra® biomembrane vs the biomembrane plus NPT. They observed significantly superior results with NPT and shorter healing times (p<0.001).25
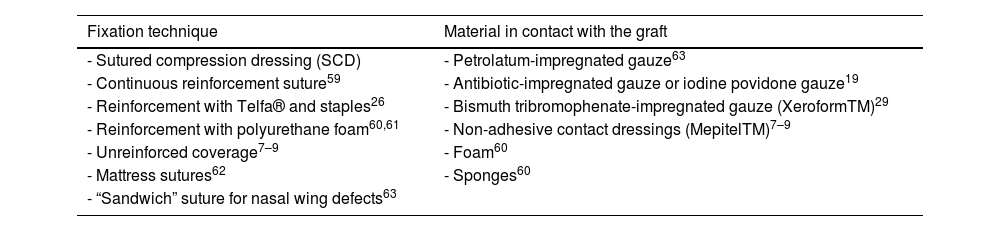
Special situationsCare of surgical wounds with skin graftsTraditionally, the tie-over bolster dressing (sutured compression dressing-SCD) has been used as a reinforcement technique. Multiple alternatives have been described (Table 5), highlighting the non-reinforced coverage, especially in grafts up to 5.5cm2, with results comparable to SCD. Advantages include shorter surgical times and increased patient comfort.26–29 The graft is covered with a non-adhesive dressing, with or without another non-adhesive contact dressing or rolled gauze impregnated over the graft. It can be secured with sterile bands, elastic bandage, or adhesive dressing.
Techniques for fixing skin grafts and materials in contact with the graft.
| Fixation technique | Material in contact with the graft |
|---|---|
| - Sutured compression dressing (SCD) | - Petrolatum-impregnated gauze63 |
| - Continuous reinforcement suture59 | - Antibiotic-impregnated gauze or iodine povidone gauze19 |
| - Reinforcement with Telfa® and staples26 | - Bismuth tribromophenate-impregnated gauze (XeroformTM)29 |
| - Reinforcement with polyurethane foam60,61 | - Non-adhesive contact dressings (MepitelTM)7–9 |
| - Unreinforced coverage7–9 | - Foam60 |
| - Mattress sutures62 | - Sponges60 |
| - “Sandwich” suture for nasal wing defects63 |
We have not found any studies directly comparing the results between different materials, or between different types of dressings after covering with skin grafts in dermatologic surgery.
Donor site wounds for skin graftsA systematic review that included 35 studies comparing different modalities of dressings in the donor site of PTSG—mostly comparing dry dressing (with gauze) vs advanced dressings—reported better pain control and faster healing in the latter group.30 Two previous systematic reviews showed similar results.31,32 A multicenter RCT with 288 patients with donor site defects, mainly from the thigh and > 10cm2 compared alginate dressings, films, gauze, hydrocolloids, Hydrofiber®, and silicone. They observed that re-epithelialization time with hydrocolloid dressings was 7 days shorter vs other dressings. The rate of SWI with gauze was twice that of other dressings (RR, 2.38; CI, 1.14-4.99).33
Wounds covered with dermal xenograftsBiomembranes or dermal xenografts are used to cover complex defects. They promote neovascularization of the bed, which will later facilitate re-epithelialization by secondary intention, coverage with skin grafts, or flaps. This process usually takes 14 to 21 days. The biomembrane should be kept covered by an external protective layer, usually silicone. We have not found any clinical studies yet on the type of dressings to be performed during this period. Commercial brands recommend occlusive coverage with antimicrobials for the first 5 to 7 days, which is similar to a skin graft. Contact of the graft with enzymatic debridement agents such as collagenase is ill-advised.34
Surgical wounds with secondary intention healingWounds with secondary intention healing (SIH) after dermatological surgery are not associated with a higher risk of SWI than direct closure, according to the results of a recent systematic review.35 In an extensive Cochrane review (14 centers, n=886), a reduction in the risk of SWI in SIH with topical antibiotics was suggested. However, the included studies were small and with different types of wounds, such as post-diabetic foot amputation, pilonidal sinus surgery and hemorrhoids, abscesses, post-C-section complications, and colostomies.36
A systematic review included 13 RCTs comparing different dressings, such as gauze, foam, powders, alginate, and hydrocolloid in SIH. The included wounds were due to the excision of pilonidal cysts, abdominal postoperative complications, and leg amputations. In general, patients experienced more pain and lower satisfaction with the use of gauze. Foam dressings appeared to be superior to conventional gauze in terms of patient satisfaction, pain reduction, and nursing care.37
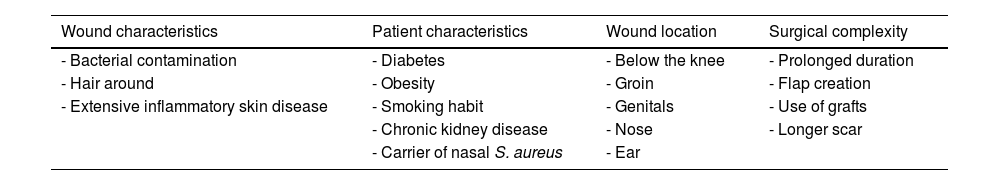
Surgical wounds on the legsMultiple studies have described a higher rate of complications after dermatological surgery below the knee38 (Table 6). Recently, a retrospective study that included 23,121 excisions in dermatological surgery proved that this location was associated with a higher risk of SWI (odds ratio [OR], 1.908; CI, 1.126-3.235) and higher rates of dehiscence (OR, 4.037; CI, 2.654-6.140), which increased significantly if the patient was ≥80 years old (OR, 9.632; CI, 5.635-16.464)1.
Factors associated with increased risk of surgical wound infection.
| Wound characteristics | Patient characteristics | Wound location | Surgical complexity |
|---|---|---|---|
| - Bacterial contamination | - Diabetes | - Below the knee | - Prolonged duration |
| - Hair around | - Obesity | - Groin | - Flap creation |
| - Extensive inflammatory skin disease | - Smoking habit | - Genitals | - Use of grafts |
| - Chronic kidney disease | - Nose | - Longer scar | |
| - Carrier of nasal S. aureus | - Ear |
Studies on post-dermatologic surgery dressings on the legs are scarce. We found 2 retrospective studies in which zinc oxide and compression was used. A recent study (n=80) evaluated the time until complete healing of the surgical wound with primary closure in individuals undergoing excision of skin lesions on the legs. They compared a group with conventional dressings—gauze and non-adhesive dressings—vs a different group with gauze impregnated with zinc oxide and compression with an elastic bandage (the dressing was changed weekly). By day 19, 66% of patients from the first group and 92% of the zinc oxide+compression group had achieved complete healing (p <0.001). A total of 14% of the group with conventional dressings had complications vs 0 individuals from the zinc oxide+compression group.12 Similar results were found in a small clinical trial (n=10) of patients with surgical wounds left to heal by secondary intention using a Unna boot (a bandage with zinc oxide-impregnated wraps). The dressing was changed weekly.39
For the role of compression, a recent systematic literature review40 found 2 studies evaluating its role in post-dermatologic surgery dressings: the above-mentioned clinical trial using the Unna boot,39 and a retrospective study (n=366) that revealed that pre- and postoperative compression was associated with a statistically nonsignificant lower rate of complications (OR, 0.67).41
DiscussionIn general, the evidence on dressings after dermatological surgery is limited. Some recommendations come from other specialties, which perform procedures that may have higher rates of complications, and therefore, these recommendations may not be generalizable.
We found a wide variability of postoperative recommendations among dermatologists. A study analyzed a total of 169 care protocols from 119 centers, mostly American. A total of 84% recommended the application of petroleum-based products, specifically petrolatum (75%) and Aquaphor® (43%), 43% indicated topical antibiotics, and 24% discouraged them.5 A recent survey of 196 dermatologic surgeons proved that 95% recommended some antiseptic for surgical wound care, mainly washing with water and soap (65%), followed by other unspecified antiseptics, hydrogen peroxide, acetic acid, and chlorhexidine.42
The ideal dressing should be hemostatic, protective against infections, immobilizing, moist, and absorbent of excess exudate. Comparative studies of gauze vs advanced dressings come mainly from general surgery and chronic or traumatic wounds. In general, no differences in healing time, aesthetic outcomes, or SWI have been demonstrated.13,43 In dermatology, although evidence is limited, advanced dressings may be more comfortable for the patient and require fewer dressing changes,11 but at a higher cost.
Dermatology is one of the specialties that most prescribes antibiotics.44 The use of prophylactic topical antibiotics is not recommended for clean dermatological surgical wounds.7,8,45–48 In addition to not being beneficial, their use may be associated with bacterial resistance and the development of allergic contact dermatitis.8 Necrosis of the wound has even been reported with the use of mupirocin.46 In general, petrolatum or topical silicone is preferred instead.2,6,8,49
Despite the lack of evidence, in SIH and skin grafts, an emollient like petrolatum and an occlusive or semi-occlusive dressing may be used to prevent desiccation and infection.50 Skin grafts should remain covered for 5 to 7 days. Once the dressing has been removed, it is suggested to keep the wound hydrated and clean. A survey of 294 Mohs surgeons revealed that most recommended petroleum jelly or Aquaphor® for care (64% and 38%, respectively), and that more than 85% did not change this recommendation in SIH or grafts.51
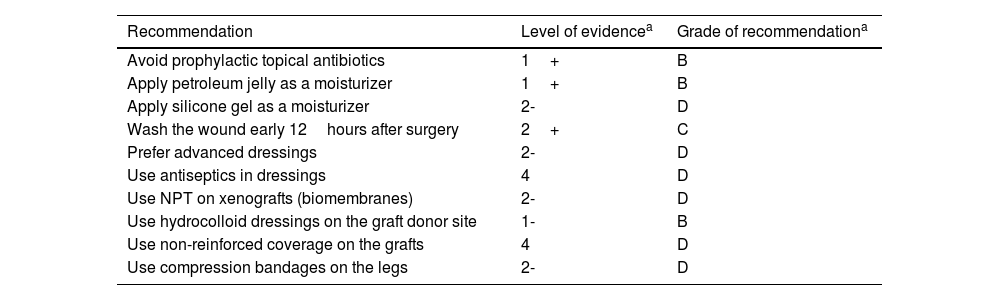
For postoperative wounds below the knee, the combination of zinc oxide and compression seems to be a good option. Although compression is widely recommended to treat venous ulcers in this location, its evidence in surgical wounds is scarce.12 In a recent study, only 7.5% of patients with dermatological surgical wounds below the knee received postoperative compression.52Table 7 illustrates recommendations and their level of evidence in the management of wounds after dermatologic surgery.
Recommendations for managing wounds after dermatological surgery.
| Recommendation | Level of evidencea | Grade of recommendationa |
|---|---|---|
| Avoid prophylactic topical antibiotics | 1+ | B |
| Apply petroleum jelly as a moisturizer | 1+ | B |
| Apply silicone gel as a moisturizer | 2- | D |
| Wash the wound early 12hours after surgery | 2+ | C |
| Prefer advanced dressings | 2- | D |
| Use antiseptics in dressings | 4 | D |
| Use NPT on xenografts (biomembranes) | 2- | D |
| Use hydrocolloid dressings on the graft donor site | 1- | B |
| Use non-reinforced coverage on the grafts | 4 | D |
| Use compression bandages on the legs | 2- | D |
NPT, negative pressure therapy.
In general, patients follow post-dermatologic surgery wound care instructions. However, a significant number of patients—especially the elderly—have difficulty understanding them, highlighting the need to explain them adequately and adapt them to their social reality.53,54
LimitationsThis review is limited by being a narrative and not a systematic literature review. The studies included are methodologically heterogeneous, and in many cases, there are no comparative studies across the various agents/procedures. Furthermore, clinical trials on the topic are scarce.
ConclusionsThere is significant variability in postoperative wound care recommendations in dermatology. The evidence on the superiority of different agents and techniques is, in most cases, limited. However, there is sufficient evidence to discourage the use of prophylactic topical antibiotics. The use of petrolatum or silicone gel offers similar results, with lower complication rates. Although advanced dressings may be more comfortable than conventional ones due to less frequent changes, they are not superior in terms of healing time or incidence of SWI. In SIH, foam dressings appear to be superior to conventional gauze. Prospective comparative studies are needed to provide evidence-based recommendations.
FundingNone declared.
Conflicts of interestNone declared.