PRP is a rare entity of unknown etiopathogenesis. Lack of management guidelines makes it a challenge for clinicians.
ObjectiveTo add our experience to increase evidence about PRP.
MethodsWe performed a retrospective, descriptive and multicentric study of 65 patients with PRP, being the largest European case series of patients with PRP.
ResultsPRP was more frequent in male patients with an average age of 51 years, but erythrodermic forms presented in older patients (average age 61 years).
Six (75%) paediatric patients and ten (60%) non-erythrodermic adults controlled their disease with topical corticosteroids. On the contrary, 26 (68%) erythrodermic patients required biologic therapy as last and effective therapy line requiring an average of 6.5 months to achieve complete response.
ConclusionOur study showed a statistical difference in terms of outcome and response to treatment between children or patients with limited disease and patients who develop erythroderma.
La pitiriasis rubra pilaris (PRP) es una entidad rara de etiopatogenia desconocida. La falta de guías clínicas la convierte en un desafío para los clínicos.
ObjetivoContribuir con nuestra experiencia a aumentar la evidencia disponible sobre esta entidad.
MétodoRealizamos un estudio retrospectivo, descriptivo y multicéntrico de 65 pacientes con PRP, siendo la serie de casos europea más grande de pacientes con PRP.
ResultadosLa PRP fue más frecuente en los varones con una edad promedio de 51 años, pero las formas eritrodérmicas se presentaron en pacientes de mayor edad, en torno a los 61 años.
Seis (75%) de los pacientes pediátricos y 10 (60%) de los adultos no eritrodérmicos controlaron su enfermedad con corticoides tópicos. Por el contrario, 26 (68%) de los pacientes eritrodérmicos necesitaron terapia biológica como última línea terapéutica eficaz; requiriendo un promedio de 6,5 meses para lograr una respuesta completa.
ConclusiónNuestro estudio mostró una diferencia estadística en términos de resultado y respuesta al tratamiento entre niños o pacientes con enfermedad limitada y pacientes que desarrollan eritrodermia.
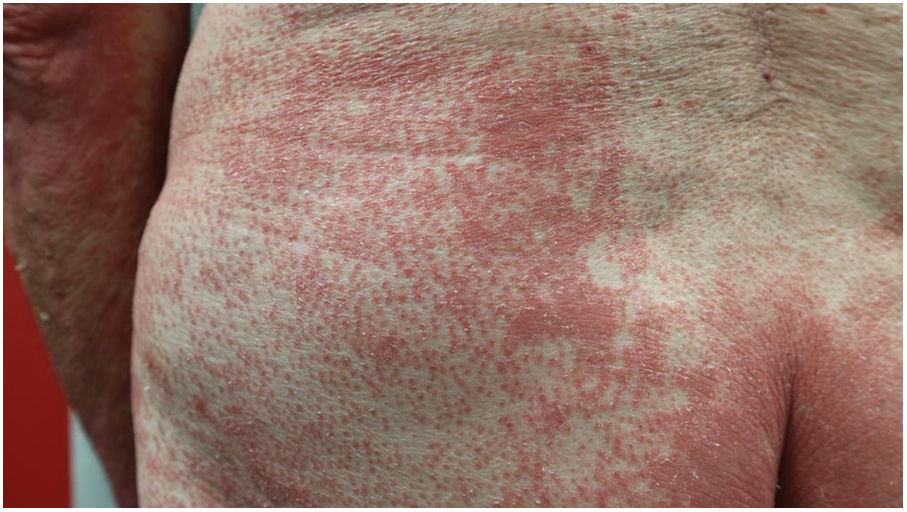
Pityriasis rubra pilaris (PRP) is an uncommon inflammatory disease, traditionally considered as a reactive dermatosis.1–3 It appears in a bimodal age distribution in the first and fifth decades of life and equal frequency between sexes, as follicular hyperkeratotic salmon-coloured papules and plaques. Some patients develop erythroderma leaving islands of non-involved skin.1,2
Diagnosis requires a good clinicopathological correlation. Alternating orthokeratosis and parakeratosis known as “checkerboard pattern” is the main histopathological feature. Treatment options include a variety of drugs such as topical corticosteroids, oral retinoids, immunosuppressors and biologic agents.2,4–6 PRP is believed to share cytokine environment with psoriasis.7
There are no management guidelines and current scientific evidence is limited to case reports and case series. Consequently, management of PRP represents a challenge.
ObjectiveTo describe clinicopathological and demographic features of PRP patients and analyze treatment modalities.
MethodsA multicentre retrospective observational study was carried out in eight Spanish hospitals including patients with clinicopathological diagnosis of PRP, treated during 20 years (between January 2002 and February 2022). Variables were collected by each centre in an anonymous database. Paediatric sample was defined as patients under 16 years old. We used body surface area (BSA) to evaluate treatment response and considered complete response as BSA <3%. A descriptive statistical analysis was performed, using the mean and standard deviation (SD) for quantitative variables and absolute and relative frequencies for qualitative variables. The contrasts of hypotheses in the qualitative variables were carried out using the Chi-square test or Fisher's exact test and for quantitative variables using the Student's t-test. A p-value <0.05 was considered statistically significant. All analyses were performed with the statistical software Stata® (StataCorp, College Station, Texas) version 16 for Windows. The study was conducted following the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Hospital 12 de Octubre (No: 22/233).
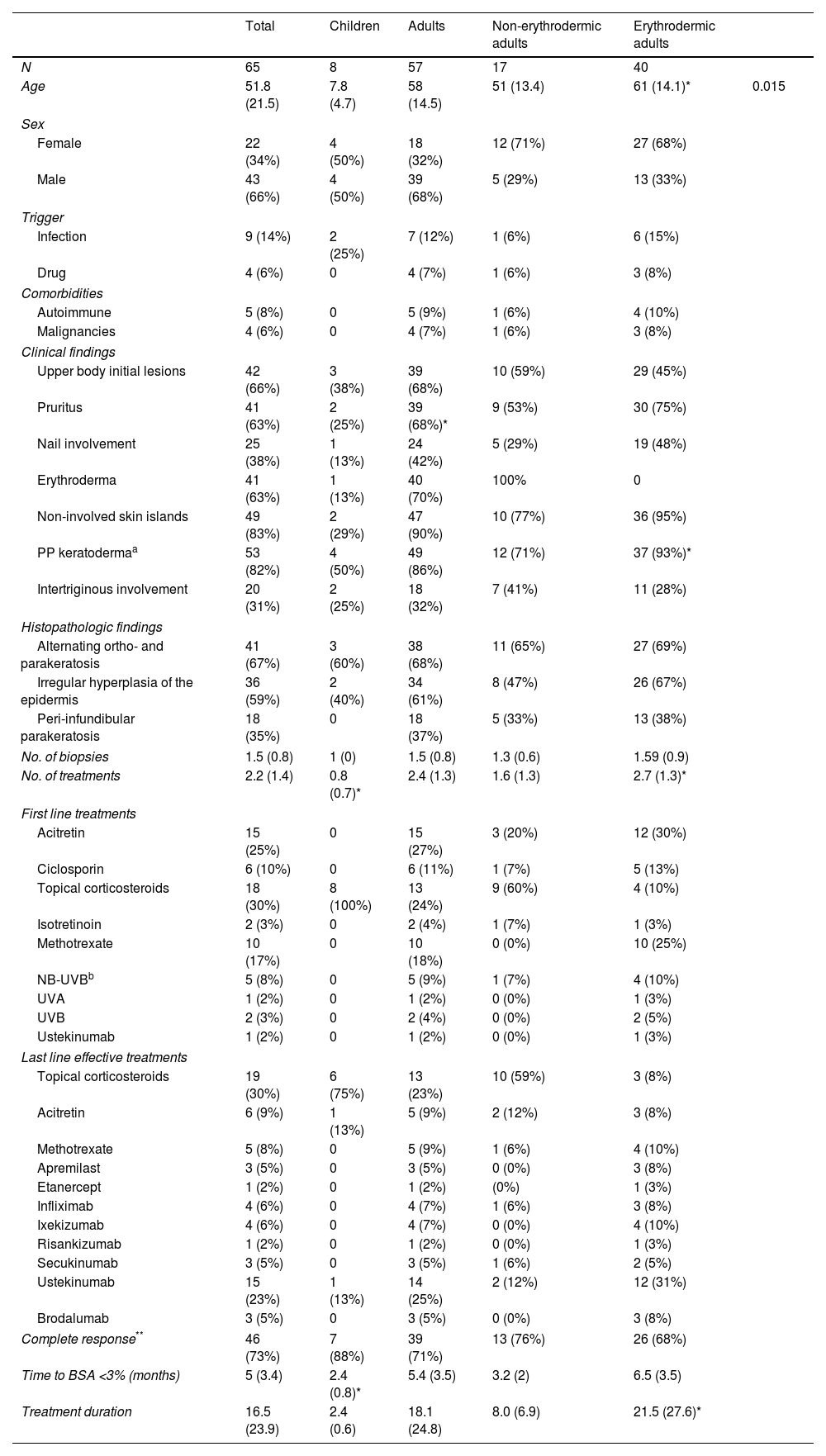
ResultsEpidemiological, clinical, histopathological and therapeutic results can be found in Table 1.
Detailed patient's characteristics.
| Total | Children | Adults | Non-erythrodermic adults | Erythrodermic adults | ||
|---|---|---|---|---|---|---|
| N | 65 | 8 | 57 | 17 | 40 | |
| Age | 51.8 (21.5) | 7.8 (4.7) | 58 (14.5) | 51 (13.4) | 61 (14.1)* | 0.015 |
| Sex | ||||||
| Female | 22 (34%) | 4 (50%) | 18 (32%) | 12 (71%) | 27 (68%) | |
| Male | 43 (66%) | 4 (50%) | 39 (68%) | 5 (29%) | 13 (33%) | |
| Trigger | ||||||
| Infection | 9 (14%) | 2 (25%) | 7 (12%) | 1 (6%) | 6 (15%) | |
| Drug | 4 (6%) | 0 | 4 (7%) | 1 (6%) | 3 (8%) | |
| Comorbidities | ||||||
| Autoimmune | 5 (8%) | 0 | 5 (9%) | 1 (6%) | 4 (10%) | |
| Malignancies | 4 (6%) | 0 | 4 (7%) | 1 (6%) | 3 (8%) | |
| Clinical findings | ||||||
| Upper body initial lesions | 42 (66%) | 3 (38%) | 39 (68%) | 10 (59%) | 29 (45%) | |
| Pruritus | 41 (63%) | 2 (25%) | 39 (68%)* | 9 (53%) | 30 (75%) | |
| Nail involvement | 25 (38%) | 1 (13%) | 24 (42%) | 5 (29%) | 19 (48%) | |
| Erythroderma | 41 (63%) | 1 (13%) | 40 (70%) | 100% | 0 | |
| Non-involved skin islands | 49 (83%) | 2 (29%) | 47 (90%) | 10 (77%) | 36 (95%) | |
| PP keratodermaa | 53 (82%) | 4 (50%) | 49 (86%) | 12 (71%) | 37 (93%)* | |
| Intertriginous involvement | 20 (31%) | 2 (25%) | 18 (32%) | 7 (41%) | 11 (28%) | |
| Histopathologic findings | ||||||
| Alternating ortho- and parakeratosis | 41 (67%) | 3 (60%) | 38 (68%) | 11 (65%) | 27 (69%) | |
| Irregular hyperplasia of the epidermis | 36 (59%) | 2 (40%) | 34 (61%) | 8 (47%) | 26 (67%) | |
| Peri-infundibular parakeratosis | 18 (35%) | 0 | 18 (37%) | 5 (33%) | 13 (38%) | |
| No. of biopsies | 1.5 (0.8) | 1 (0) | 1.5 (0.8) | 1.3 (0.6) | 1.59 (0.9) | |
| No. of treatments | 2.2 (1.4) | 0.8 (0.7)* | 2.4 (1.3) | 1.6 (1.3) | 2.7 (1.3)* | |
| First line treatments | ||||||
| Acitretin | 15 (25%) | 0 | 15 (27%) | 3 (20%) | 12 (30%) | |
| Ciclosporin | 6 (10%) | 0 | 6 (11%) | 1 (7%) | 5 (13%) | |
| Topical corticosteroids | 18 (30%) | 8 (100%) | 13 (24%) | 9 (60%) | 4 (10%) | |
| Isotretinoin | 2 (3%) | 0 | 2 (4%) | 1 (7%) | 1 (3%) | |
| Methotrexate | 10 (17%) | 0 | 10 (18%) | 0 (0%) | 10 (25%) | |
| NB-UVBb | 5 (8%) | 0 | 5 (9%) | 1 (7%) | 4 (10%) | |
| UVA | 1 (2%) | 0 | 1 (2%) | 0 (0%) | 1 (3%) | |
| UVB | 2 (3%) | 0 | 2 (4%) | 0 (0%) | 2 (5%) | |
| Ustekinumab | 1 (2%) | 0 | 1 (2%) | 0 (0%) | 1 (3%) | |
| Last line effective treatments | ||||||
| Topical corticosteroids | 19 (30%) | 6 (75%) | 13 (23%) | 10 (59%) | 3 (8%) | |
| Acitretin | 6 (9%) | 1 (13%) | 5 (9%) | 2 (12%) | 3 (8%) | |
| Methotrexate | 5 (8%) | 0 | 5 (9%) | 1 (6%) | 4 (10%) | |
| Apremilast | 3 (5%) | 0 | 3 (5%) | 0 (0%) | 3 (8%) | |
| Etanercept | 1 (2%) | 0 | 1 (2%) | (0%) | 1 (3%) | |
| Infliximab | 4 (6%) | 0 | 4 (7%) | 1 (6%) | 3 (8%) | |
| Ixekizumab | 4 (6%) | 0 | 4 (7%) | 0 (0%) | 4 (10%) | |
| Risankizumab | 1 (2%) | 0 | 1 (2%) | 0 (0%) | 1 (3%) | |
| Secukinumab | 3 (5%) | 0 | 3 (5%) | 1 (6%) | 2 (5%) | |
| Ustekinumab | 15 (23%) | 1 (13%) | 14 (25%) | 2 (12%) | 12 (31%) | |
| Brodalumab | 3 (5%) | 0 | 3 (5%) | 0 (0%) | 3 (8%) | |
| Complete response** | 46 (73%) | 7 (88%) | 39 (71%) | 13 (76%) | 26 (68%) | |
| Time to BSA <3% (months) | 5 (3.4) | 2.4 (0.8)* | 5.4 (3.5) | 3.2 (2) | 6.5 (3.5) | |
| Treatment duration | 16.5 (23.9) | 2.4 (0.6) | 18.1 (24.8) | 8.0 (6.9) | 21.5 (27.6)* | |
A total of 65 patients were included. At the time of enrolment, eight children were an average of 7.8 years old and four (50%) were female.
Thirty-nine male patients represented 68% of the adult sample and 40 of them (70%) developed erythroderma, but only one child. Mean age was older in erythrodermic adults (61 years) when compared to non-erythrodermic adults (51 years, p-value=0.015).
Triggers and comorbiditiesA possible trigger was reported by 13 (20%) patients: previous infection in seven (12%) adults and two (25%) children; and drugs in four (7%) adults.
Infections in children were all upper airway infections. Among adult patients we did not collect specific microbiological agents data.
Four (7%) of our adult patients had concomitant neoplasia and five (9%) autoimmune comorbidities.
Clinical findingsThe most frequent clinical features were non-involved skin islands (n=49, 83%) and palmoplantar (PP) keratoderma (n=53, 85%), which were also more frequent in erythrodermic patients (Figs. 1 and 2). The difference was statistically significant for PP keratoderma (p-value 0.029). However, children presented only in two (29%) and four (50%) of the cases with non-involved skin islands and PP keratoderma respectively.
Forty-one (63%) of patients reported pruritus, but only two (25%) children and 39 (68%) of the adults (p-value 0.017).
Other clinical features are detailed in Table 1.
HistologyMost frequent histological findings were alternating ortho- and parakeratosis (n=41, 67%) and irregular epidermic hyperplasia (n=36, 59%).
TreatmentMean treatment duration was 2.4 months in children, 8 months in non-erythrodermic adults and 21.4 months in erythrodermic adults. Time to BSA <3% was shorter and number of treatments lower in children and non-erythrodermic adults than their counterparts (Table 1).
All of the children were initially treated with topical corticosteroids. Only one of them required acitretin. One developed erythroderma and needed ustekinumab to control the disease. Complete response (CR) was achieved in seven (88%) of the children.
Non-erythrodermic adults (n=17, 30%) were successfully managed with topical therapy in nine (60%) patients. Six were treated with systemic agents (acitretin in three and phototherapy in one patient). Only five patients required a biologic agent. CR was achieved in 13 (76%) of non-erythrodermic adults.
On the other hand, 29 (71%) erythrodermic patients were initially managed with systemic agents such as acitretin in twelve (30%) of the cases or methotrexate in ten (25%) of the patients, phototherapy in seven (18%) and one patient began with ustekinumab. Last effective treatment included a variety of drugs (Table 1) and complete response was achieved in 26 (68%) of patients. Biologic therapy was required at some point by 31 (68%) of patients and ustekinumab was the most frequently used.
LimitationsOur study has several limitations. First, our data was collected retrospectively with a relatively small sample size. In addition, there are no standardized diagnostic criteria of PRP or severity scales to asses treatment response. Lastly, the availability of new biologic therapies over the course of the last decade may have exerted some influence on therapeutic results of patients diagnosed in the latest years.
DiscussionThis study presents detailed demographic, clinicopathologic and treatment data in 65 patients diagnosed of PRP. To the best of our knowledge, this is the largest European case series of patients with PRP.
Our study demonstrated predominance in adult male patients (n=39, 68%), which differs from data on literature maintaining equal sex distribution.1–4 Bimodal distribution was consistent with literature,1–4 but strikingly adult patients presenting with erythroderma were on average 10 years older (p-value <0.05).
Multiple case reports claim possible causality between malignancies or autoimmune disorders and PRP.1,7 However, our adult patients showed a low frequency of autoimmune comorbidities (9%) and malignancies (6%), suggesting that their frequency may not differ from general population and screening procedures should be guided by history and physical examination.
Infectious and pharmacological agents have usually been described to trigger PRP.1,2 Interestingly, 25% of our children reported an infectious trigger but in only 6% of erythrodermic adults a culprit drug was demonstrated. This supports the idea of PRP as a multifactorial disease, in which environmental factors play a role but are not necessary for the development of the disease.
The most frequent histological feature was alternating ortho- and parakeratosis, matching literature data. However, histology can be unspecific and often diagnosis is predominantly guided by characteristic clinical features.1–4 Sixty-six percent of our patients initiated lesions on the upper body, which is consistent with the cephalocaudal progression classically described.1–4 Interestingly, 94% of our erythrodermic patients presented PP keratoderma (p-value 0.02).
Fouarge et al.,8 already proposed PP keratoderma as a possible factor for resistance to an IL-23/p19 antagonist. In our opinion, it may be a progression marker, and could help identify patients at a higher risk of developing erythrodermic forms of PRP.
Seventy-five percent of the paediatric patients and 60% of the non-erythrodermic adults controlled their disease with topical corticosteroids. Good response to topical therapy was already described in paediatric PRP,9 but we assume it is extendible to adults with localized disease. However, most of erythrodermic patients (68%) required biologic therapy as last and effective therapy line. Among them, 68% achieved complete response but after an average of 6.5 months of treatment. They usually receive more lines of treatment (mean 2.7) than patients with localized lesions and may benefit from a more invasive and early approach.
ConclusionIn conclusion, our study reported that PRP may be more frequent in men and erythrodermic forms could appear in older patients. There was no clear association with cancer or autoimmune diseases in our patients. PP keratoderma may be a marker of progression to erythroderma. Children and adults with localized disease in our sample usually respond to topical corticosteroids. On the contrary, erythrodermic patients received multiple therapy lines, needed an average of 6.5 months and a biologic agent to control their disease. Further studies are required to investigate this challenging disease.
Patient's consentThe patients in this manuscript have given written informed consent to publication of their case details.
FundingNone declared.
Conflicts of interest- -
Dr Ruiz Genao has been reimbursed by Almirall, Pfizer, Janssen, Amgen, Abbvie, Lilly, UCB, Novartis and Leo-Pharma for advisory services and conferences.
- -
L. Puig has perceived consultancy/speaker's honoraria from and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB.
The authors confirm that the manuscript has been submitted solely to this journal and is not submitted, in press, or published in any language elsewhere. Each author has participated sufficiently in the work to take public responsibility of the content.