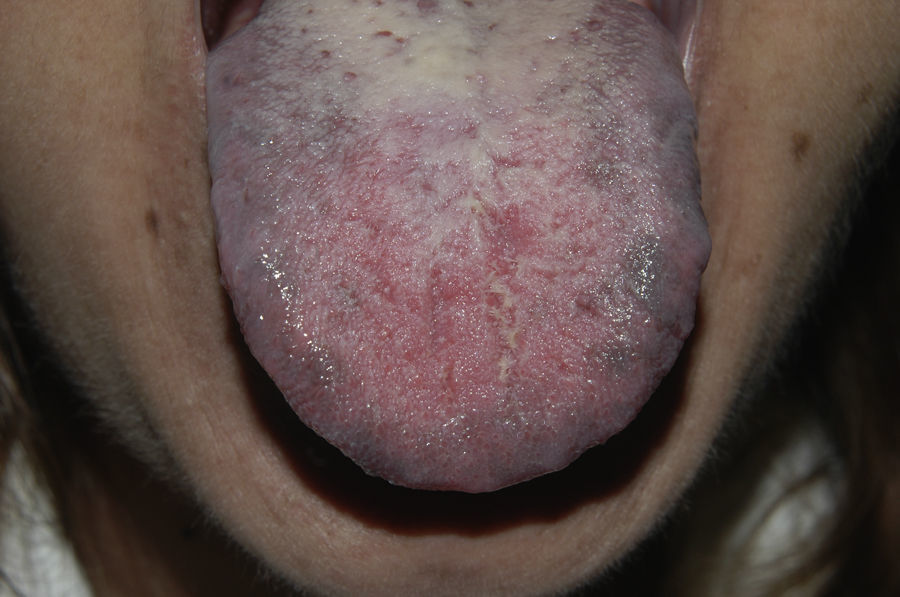
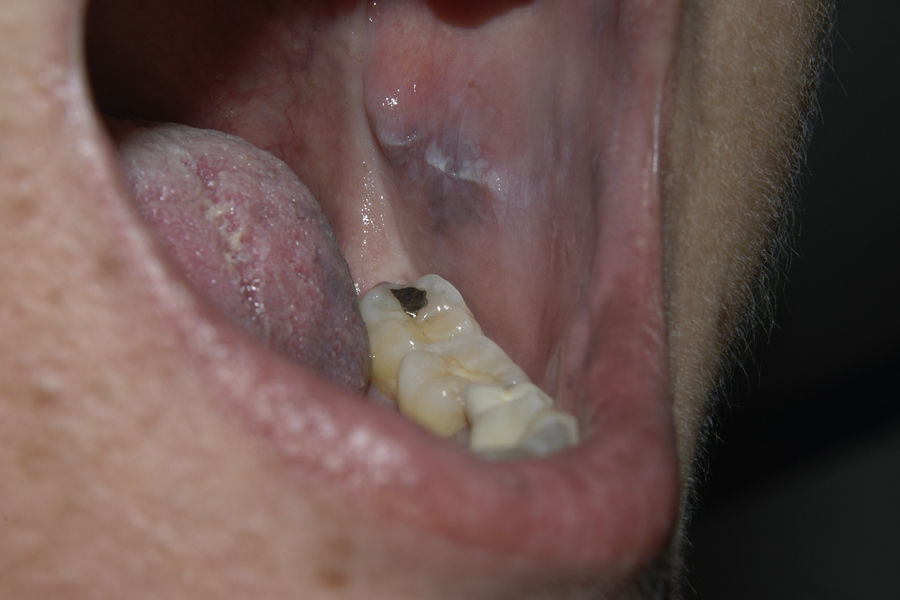
Since 2003, there have been reports of oral hyperpigmentation associated with interferon alfa and ribavirin therapy for hepatitis C. We report a new case of oral pigmentation associated with this therapy in a white woman who also developed genital mucosal lesions. The patient was 49 years old and had a history of allergy to acetylsalicylic acid, depressive disorder, and chronic hepatitis C genotype 1 infection, diagnosed in 2007. In May 2008, she started treatment with pegylated interferon alfa and ribavirin with suboptimal therapeutic adherence until November 2009. The virus was not eradicated. In June 2010, the patient presented with leukocytoclastic vasculitis and arthralgia associated with cryoglobulinemia, and received treatment with colchicine and pulses of oral prednisone. From April 2011, she underwent 5 sessions of plasmapheresis and treatment with pegylated interferon alfa (180μg/week) and ribavirin (1000mg/d) was restarted. In July 2011, she received treatment with rituximab (2 doses) with resolution of the leukocytoclastic vasculitis. In April 2011, 1 month after the start of the second course of pegylated interferon alfa and ribavirin, the patient presented with a burning sensation on the tongue and noted pigmentation of the oral and genital mucosa. In the examination, pigmented grey-blue macules were seen located mainly on the lateral areas of the dorsum of the tongue (Fig. 1). Pigmentation was also observed on both cheek mucosae (Fig. 2) and similar lesions were detected on the vulvar mucosa. The patient did not have any hyperpigmented cutaneous lesions, and no systemic symptoms were apparent at the time. Withdrawal of treatment was not required and the lesions have remained stable until present, despite continuing with treatment.
Hyperpigmentation of oral mucosa associated with interferon alfa and ribavirin combination therapy for hepatitis C was first described by Willems et al.1 in 2003. Since then, 20 cases of patients with pigmentation of oral mucosa associated with interferon alfa and ribavirin therapy have been reported, including the present case (13 women and 7 men in total).1–9 Only 6 of the 20 cases reported correspond to white patients. The first 2 cases were caused by nonpegylated interferon alfa whereas the remaining cases were associated with the pegylated formulation. The lesions appeared between 1 and 11 months after starting treatment. In 7 of the 20 cases (the present case included), the patients reported subjective symptoms.3,6–9 All cases had pigmented grey-blue macules on the lingual mucosa and most were located predominantly on lateral areas of the dorsum of the tongue. Four patients had lesions in other areas of the oral mucosa. In 5 cases, hyperpigmented cutaneous lesions were present.1–9 The presence of vulvar pigmentation has only been reported in our patient.
The development of lingual pigmentation during treatment with interferon alfa and ribavirin is not associated with any specific genotype of hepatitis virus C, dose or duration of interferon or ribavirin treatment, or response to treatment.2 Pigmentation usually increases up to the end of treatment and tends to partially resolve after discontinuation of treatment.2,8 However, there are no reports of complete resolution of lesions in the long-term. Although oral lesions may cause subjective discomfort, this is not severe and it is not recommended to discontinue treatment.3
With regard to the pathogenesis, given that interferons are able to upregulate expression of melanocyte stimulating hormone (MSH) receptors in melanocytes, Willems et al.1 suggest that interferon alfa may have been responsible for the lingual pigmentation through an increase in melanin production in the presence of MSH. It has also been suggested that pegylated interferon alfa, with a longer half-life, might increase the risk of developing oral pigmentation.4 In addition, the combination of interferon alfa with ribavirin has been associated with more adverse cutaneous reactions than administration of interferon alfa alone.10 These reactions are predominantly of the lichenoid type, suggesting a synergistic effect between the 2 drugs when inducing cutaneous side effects. The histologic image described by Willems et al.,1 with presence of pigmentary incontinence, does not rule out a probable prior lichenoid reaction, which could be another possible pathogenic mechanism. Given that postinflammatory hyperpigmentation is more frequent in dark-skinned individuals, this may explain why lesions are more common in such individuals.2
Although they appear more often in individuals with more darkly pigmented skin, lingual pigmentation due to interferon alfa and ribavirin has also been reported in white patients. This adverse reaction can be alarming for patients. Thus, candidates for interferon alfa and ribavirin therapy should be informed of the possibility of developing this type of lesion before starting treatment.
Please cite this article as: Marcoval J, Notario J, Martín C, Gómez S. Pigmentación oral asociada al tratamiento con alfa-interferón y ribavirina para la hepatitis C. Actas Dermosifiliogr. 2014;105:211–212.