Since their discovery, biologic agents have been continuously introduced into the standard treatment of inflammatory diseases with excellent therapeutic results. In spite of their evident advantages, diverse side effects arose with their use. To date, the extent to which the multiple molecular pathways overlap has yet to be thoroughly elucidated, however it is argued that the inhibition of one pathway may result in the exacerbation of antagonistic routes, inevitably leading to the onset of varied secondary pathologies.
Secukinumab, the most widely used agent of its group for the treatment of diverse inflammatory pathologies, is a monoclonal anti-IL-17A antibody. Its selective targeting and inhibition of IL-17A may cause specific symptoms due to exacerbation of antagonistic pathways. In such context, we report three cases of patients treated with secukinumab who developed nonscarring alopecia.
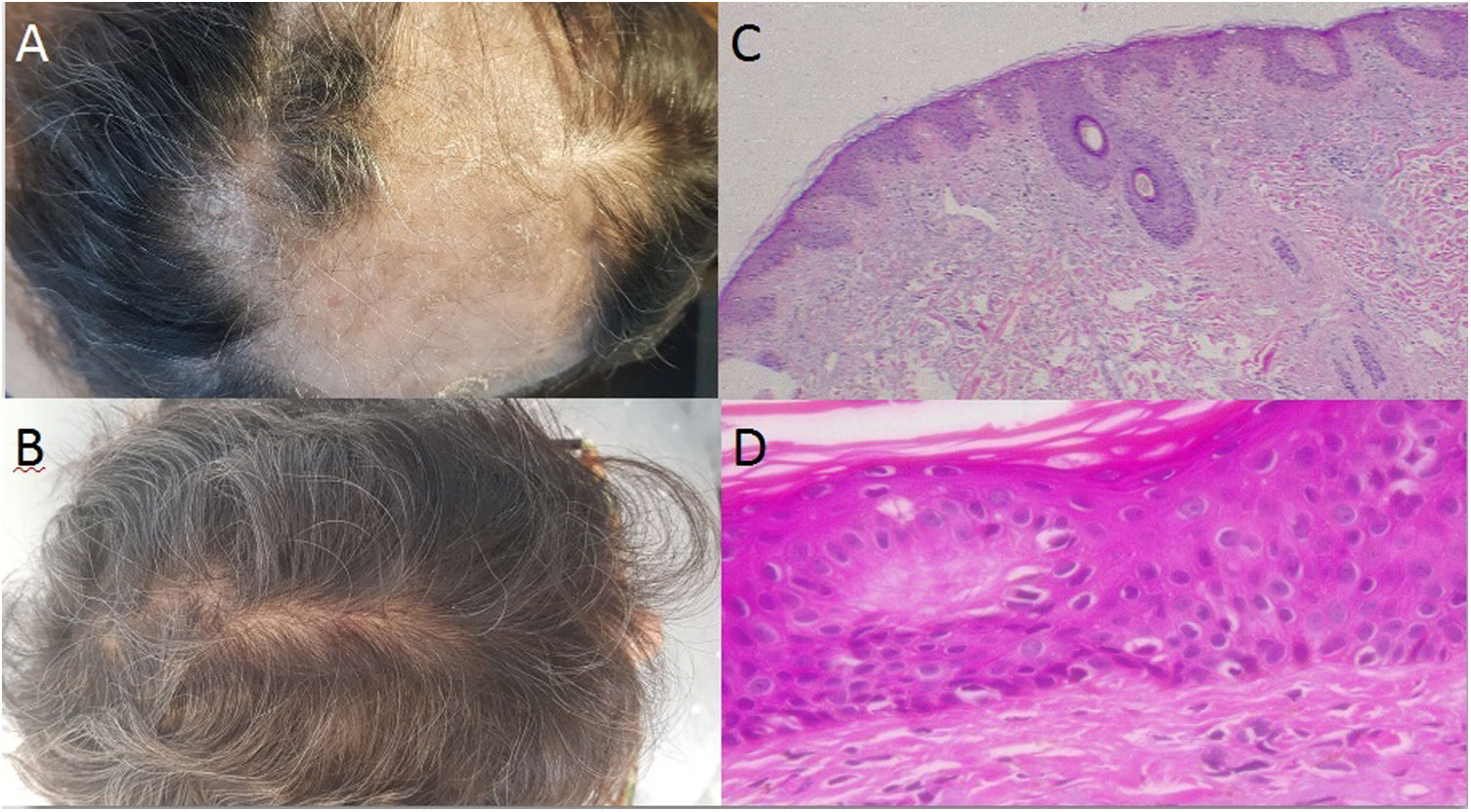
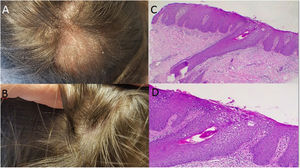
Case 1: A 58 year-old woman, with seronegative arthritis who received several treatments, including methotrexate, leflunomide, etanercept/leflunomide, adalimumab (discontinued secondary to the development of psoriatic lesions), and finally secukinumab therapy with good response. Around the 16th week of treatment the patient reported abrupt hair loss. Physical examination revealed a large area of alopecia along the parietal–temporal–occipital region with white to yellowish scales (Fig. 1A). No associated symptoms were observed and the Pull Test was negative. Two skin biopsies were performed and systemic methylprednisolone was prescribed. In both samples, histopathology revealed vacuolar interphase dermatitis, which can be related to adverse drug reaction (Fig. 1B and C), therefore, secukinumab was discontinued. The patient began receiving tofacitinib instead and full hair growth was observed after 10 months (Fig. 1D).
A: Large area of alopecia along the parietal-temporal-occipital region; with white to yellowish scales; B: Evidence of hair regrowth after the discontinuation of the treatment with secukinumab; C-D: Hematoxylin-eosin stain: Vacuolar interphase dermatitis assumed as a pharmacodermia due to the biological treatment (c x4, d x100).
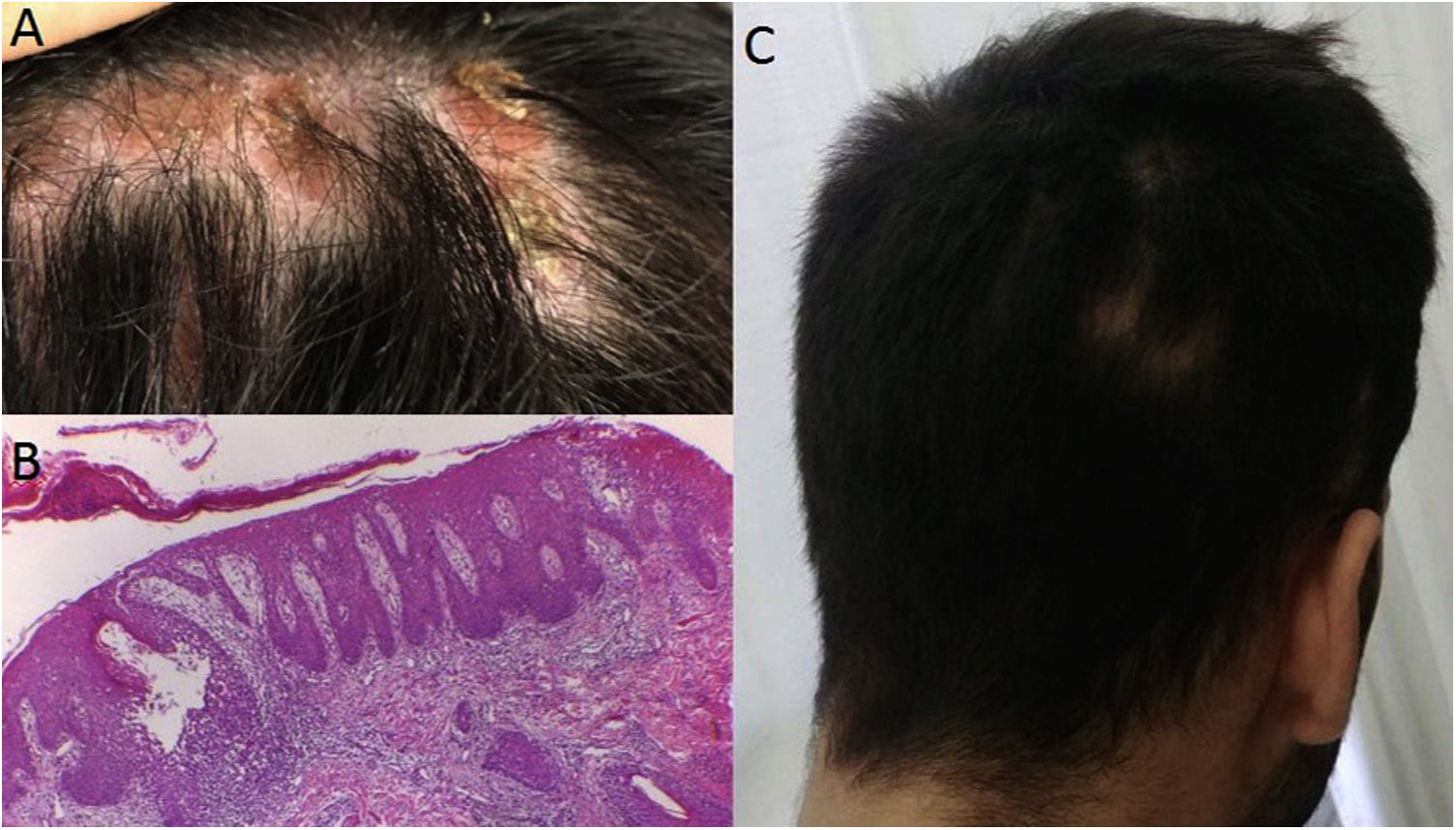
Case 2: A 25 year-old male with past medical history of ankylosing spondylitis underwent 3 months of therapy with etanercept followed by adalimumab for 3 years when a paradoxical pustular psoriasis affected palms and soles, forcing its discontinuation. Further treatment with secukinumab resulted in the onset of focal scalp alopecia by week 12. On examination, there were diffuse alopecic areas with hyperkeratosis where hair bundles emerged. A sample of the alopecic scalp was taken revealing the presence of spongiotic dermatitis with involvement of the follicles, resembling a psoriasiform pattern, with keratinocyte apoptosis and follicular hyperkeratosis. It was interpreted as a paradoxical adverse drug reaction. Consequently, tofacitinib was started with good clinical response.
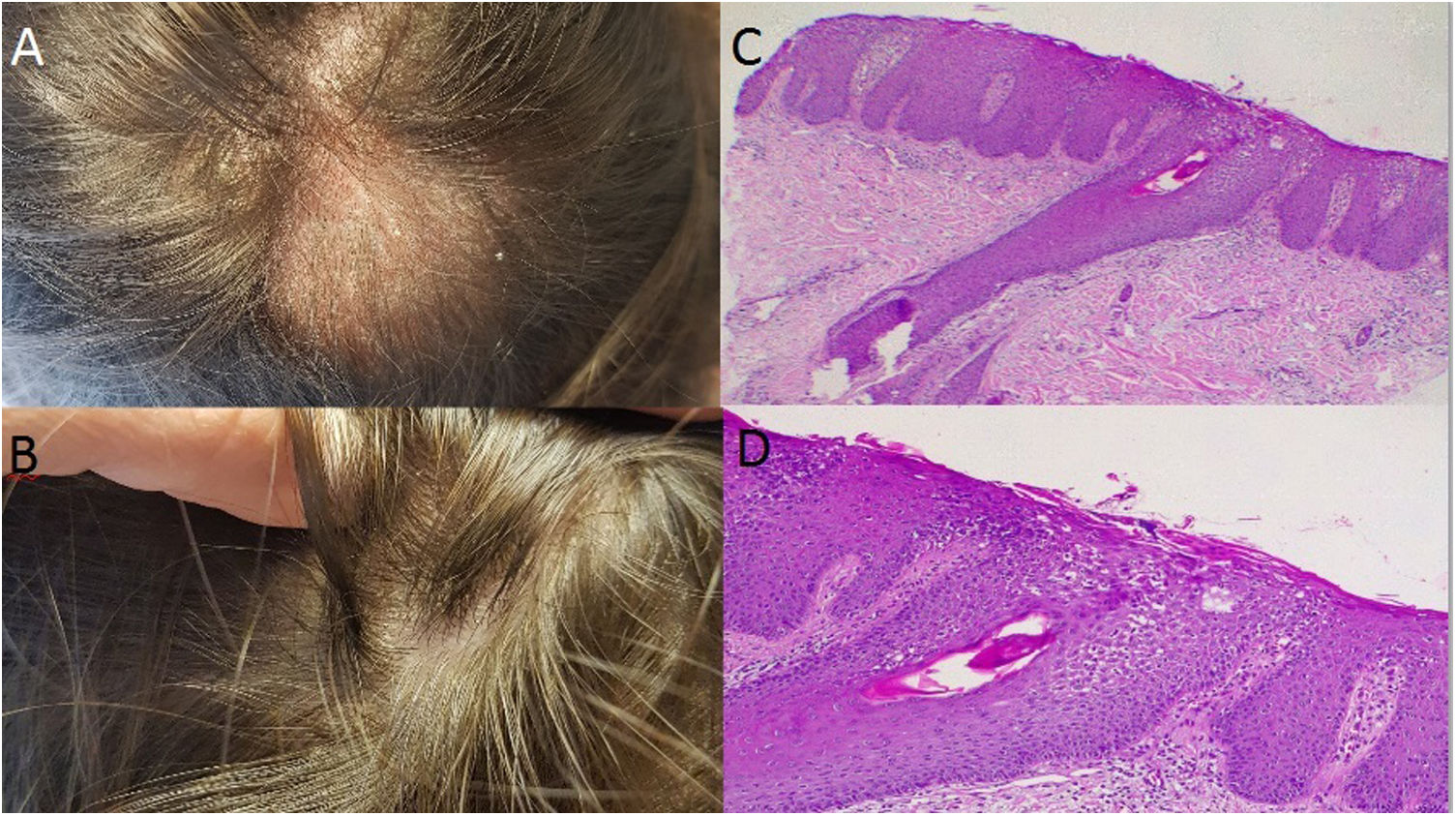
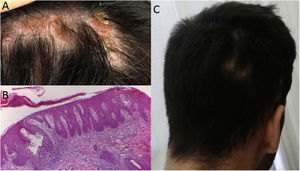
Case 3: A 37 year-old woman with large plaque psoriasis had been treated with phototherapy, methotrexate, cyclosporine and secukinumab (in the context of an investigation protocol). Successful effects were observed with the administration of secukinumab, however a fine scaled alopecic inflammatory plaque appeared around week 20 in the vertex area of the scalp (Fig. 2A). Topical treatment with clobetasol was initiated with significant improvement. Unfortunately, 4 weeks after the biological treatment was readministered the lesion recurred, presenting inflammatory signs and increased size. Secukinumab was discontinued and the skin biopsy was consistent with scalp psoriasis (Fig. 2B and C). Two months later, with almost complete clinical recovery, adalimumab was begun (Fig. 2D).
It is worth mentioning that at the time these patients consulted our service, anti-IL-23 drugs were not yet approved by national regulations as a possible treatment for psoriatic arthritis.
DiscussionAnti-IL-17A drugs are commonly used for the treatment of inflammatory diseases. We report 3 cases of nonscarring alopecia as inflammatory adverse reactions to biologic treatment.1–3 In our series this was a de novo adverse event. According to the Naranjo scale, which determines whether there is a causal relationship between an identified adverse reaction and a specific drug, the alopecia presented by our patients may have probably been related to secukinumab treatment. It is worth mentioning, that in all 3 cases the alopecia was diagnosed after 12–20 weeks of treatment with secukinumab, and progressively improved when the anti-IL-17 drug was discontinued, proving a direct relationship between the adverse event and the drug. Having ruled out other potential causes as the underlying trigger of the adverse event, by means of the histopathologic study of the lesions, the alopecia was regarded as a paradoxical adverse drug reaction.4
It should be noted that two of the patients had previous paradoxical reactions to anti-TNF agents, that did not affect the scalp. This draws attention to a possible recurrence of paradoxical adverse events related to the use of diverse biologic agents with different action mechanisms. Another patient, who had not undergone any previous anti-TNF therapy but had received secukinumab in two separate opportunities, reported alopecia as an adverse event during the second administration.
A Japanese case-report article showed similar chronological characteristics to our first and second patients, as a first treatment with adalimumab was followed by the IL-17 inhibitor brodalumab, which was discontinued due to psoriatic alopecia, interpreted by the authors as a paradoxical reaction to brodalumab.5
Alopecia manifesting as a single paradoxic reaction due to biologic treatment is unusual. Alopecia is most commonly seen associated to other dermatologic presentations, such as plaque or pustular psoriasis lesions. To date, this adverse reaction has been most commonly described with anti-TNF treatment rather than IL-17 inhibitors.6,7
ConclusionAlopecia should be considered as a paradoxical adverse drug reaction secondary to biologic therapies, especially in association with IL-17 inhibitors. Given that the histopathological findings are quite unspecific, we recommend basing the diagnosis on validated scales, such as the Naranjo scale, in order to establish a potential causal relationship between a drug and an adverse event. Our first case showed pharmacological skin changes which manifested as alopecia, whilst cases 2 and 3 revealed psoriasiform skin reactions. Based on clinical findings, we conclude that all three can be considered paradoxical adverse drug reactions associated to treatment with anti-IL-17.
Conflict of interestThe authors declare not to have any conflict of interest.