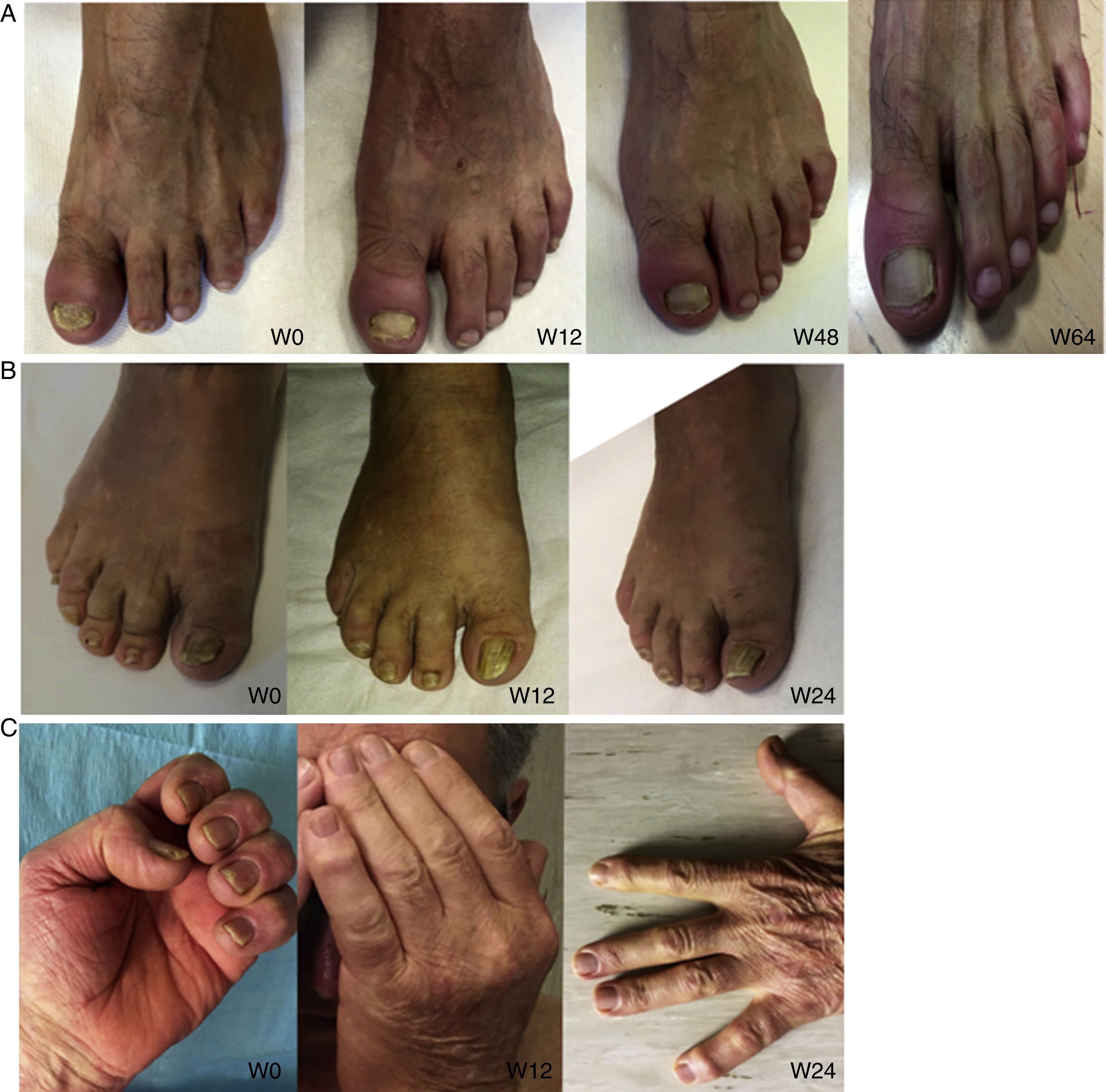
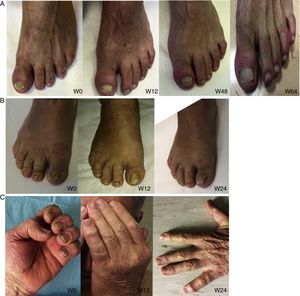
Nail psoriasis is a common complaint in psoriasis. It can be a sign of severe disease and must be taken into account when choosing a treatment aimed at reducing pain, functional disability, and emotional stress. An estimated 90% or so of patients with psoriasis will develop nail psoriasis at some stage in their lifetime, although the condition is uncommon in the pediatric population (prevalence, 7%-13%).1
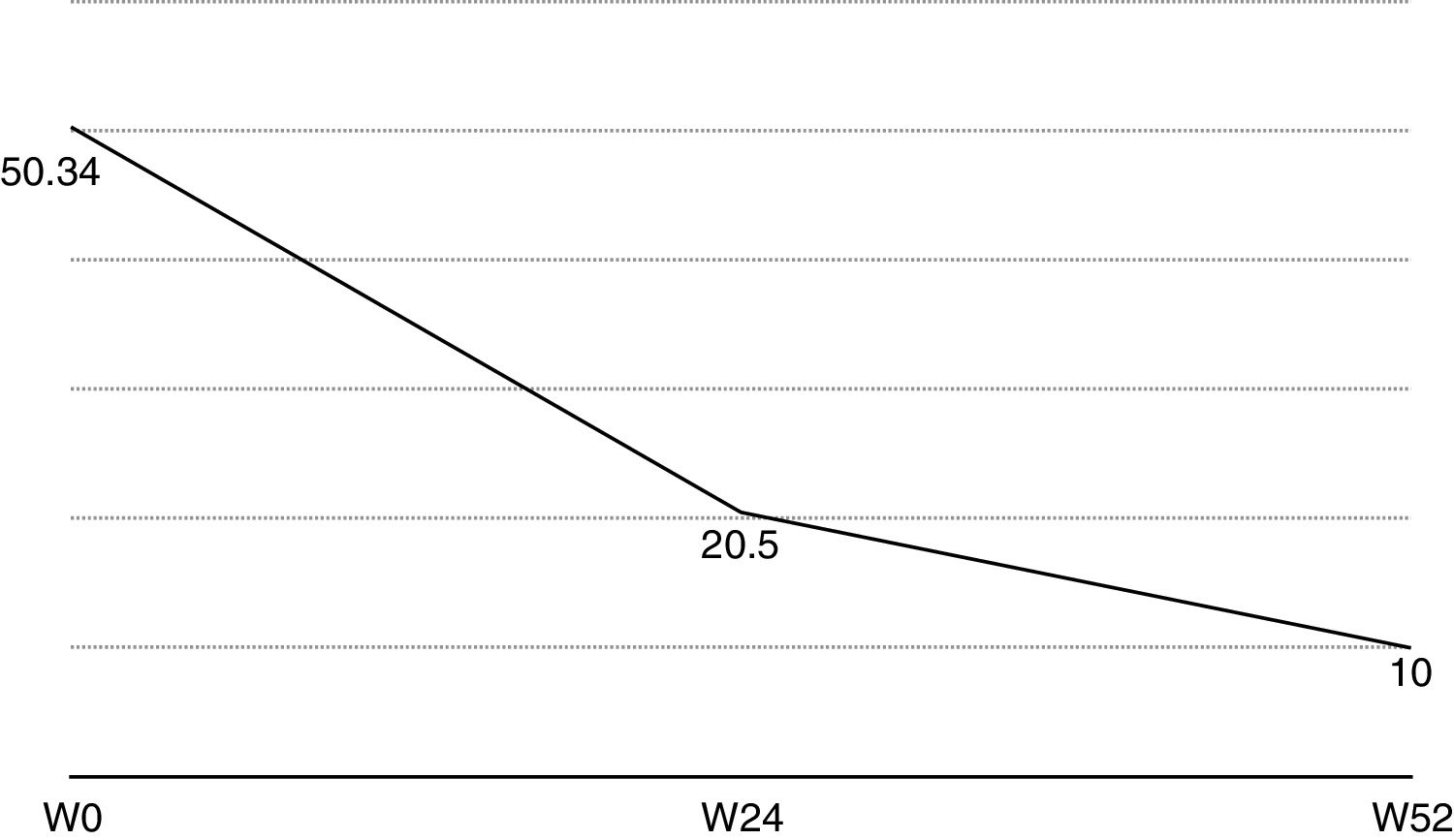
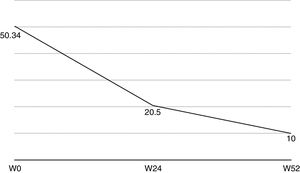
Nail psoriasis can have a major impact on patient quality of life, as it can cause intense pain or interfere with the ability to pick up small objects or perform fine motor movements. We present our experience with 8 patients (4 men and 4 women) with a mean (SD) age of 59.8 (8) years with psoriatic arthritis (PsA) and severe nail damage treated with certolizumab pegol (CZP) monotherapy using the standard dosage of 400mg at weeks 0, 2, and 4, followed by 200mg every 2 weeks. CZP is a TNF inhibitor formed by the Fab’ fragment of a humanized antibody. It was approved by the European Medicines Agency in 2009 for the treatment of active PsA in adults in whom disease-modifying antirheumatic drugs (DMARDs) do not produce an adequate response or are contraindicated. Despite our small sample, the aim of this study was to evaluate the efficacy and safety of CZP in the treatment of nail psoriasis. We included adults aged 18 years or older with a clinical and instrumental diagnosis (confirmed by ultrasound and/or magnetic resonance imaging) of nail psoriasis and PsA of over 6 months’ duration who did not respond to or tolerate conventional DMARDs, including methotrexate, azathioprine, and leflunomide. We also included patients previously treated with other biologic drugs (tumor necrosis factor and/or interleukin 12/23 inhibitors). Patients were evaluated using the Nail Psoriasis Severity Index (NAPSI), the Health Assessment Questionnaire for patients with spondyloarthritis (spA-HAQ), the Dermatology Life Quality Index (DLQI), and the Disease Activity Score based on the 44 swollen joint count (DAS-44) correlated with erythrocyte sedimentation rate (ESR) in the first hour (DAS44-ESR) at weeks 0, 24, and 52.2 The nails were also photographed at each visit to allow objective disease evaluation. The mean NAPSI score improved from 50.34 at baseline to 20.5 at week 24 and 10 at week 52 (Figs. 1 and 2). Mean DAS44-ESR also improved in patients with PsA, with a reduction from 4.4 (0.6) at baseline to 1.9 (0.5) at week 24 and 0.7 (0.5) at week 52. The mean DLQI score improved from 26 at baseline to 8 at week 24 and 5 at week 52. There was also an improvement in mean SpA-HAQ score, with a reduction from 1.65 at baseline to 0.75 at week 24 and 0.35 at week 52. Although Psoriasis Area and Severity Index assessment was not an objective in our study, we noticed a reduction in mean score from 5.1 (5.7) at baseline to 0.8 (1.2) at week 24. This score remained at 0.8 up to week 52. Improvement in nail psoriasis became evident after 4 doses (week 8). The improvement continued up to week 24 and was maintained for the rest of the year (up to week 52).
Taken together, the results of our study show that CZP improved the clinical manifestations of psoriasis over the course of 1 year and is a safe, well-tolerated treatment for refractory nail psoriasis. The efficacy and safety of CZP in the treatment of PsA has been studied in the RAPID-PsA trial. This is a phase 3, multicenter, double-blind, placebo-controlled trial in which patients were randomized to CZP 200mg every 2 weeks (Q2W), 400mg every 4 weeks (Q4W), or placebo to evaluate effects on the signs and symptoms of PsA over a period of 24 weeks. The results at 24 weeks for the group of patients with nail psoriasis at baseline (73.3%) showed a change in the modified NAPSI of −1.6 for the Q2W group and −2.0 for the QW4 group versus −1.1 for the placebo group (P=.003 and P<.001, respectively).3 Our results are consistent with other findings that have shown that CZP offers rapid and considerable improvement in psoriatic nail disease.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Mazzeo M, Dattola A, Cannizzaro MV, Bianchi L. Psoriasis ungueal tratada con certolizumab pegol en pacientes con artritis psoriásica: conclusión preliminar. Actas Dermosifiliogr. 2019;110:169–171.