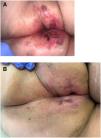
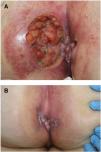
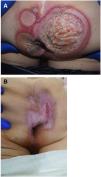
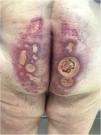
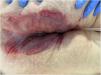
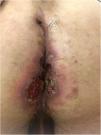
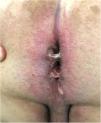
The development of perianal ulcers related to the use of a hemorrhoidal ointment has not been reported in the literature. We describe a series of 11 patients who were treated for perianal ulcers in 10 Spanish hospitals after they used the same ointment containing the active ingredients triamcinolone acetonide, lidocaine, and pentosan polysulfate sodium. No prior or concomitant conditions suggesting an alternative cause for the condition could be identified, and after the patients stopped using the ointment, their ulcers cleared completely in 8 weeks on average. This case series shows the damage that can be caused by an over-the-counter pharmaceutical product used without medical follow-up. It also illustrates the need to ask patients with perianal ulcers about any topical agents used before the lesions appeared.
La aparición de úlceras perianales en relación con una pomada antihemorroidal es una condición nunca antes reportada en la literatura. Presentamos una serie de 11 casos de 10 hospitales españoles con diagnóstico de úlceras perianales tras la aplicación de una misma pomada antihemorroidal con acetónido de triamcinolona, lidocaína y pentosano polisulfato sódico como principios activos. No se ha podido identificar ninguna condición previa o enfermedad concomitante que pudiera justificar un diagnóstico etiológico alternativo y tras retirar la pomada antihemorroidal se ha evidenciado una resolución completa de las úlceras en un periodo medio de 8 semanas. Esta serie de casos evidencia el potencial efecto dañino de un producto farmacéutico no sujeto a prescripción ni seguimiento médico y la necesidad de interrogar por el uso de agentes tópicos ante la aparición de úlceras perianales.
Perianal ulcers can develop in relation to a wide variety of causes (Table 1). When ulcers form during the use of pharmaceutical products, poor perfusion of the perianal region seems to be the main underlying mechanism, as has been reported after the use of vasoconstrictor-containing suppositories1 or nicorandil.2 Corticosteroids have also been associated with ulcers in this region3 and at other inter-triginous sites.4 To date the literature does not offer cases of perianal ulcers related to the use of a product for hemorrhoids. We report 11 cases of these ulcers forming after the use of the same hemorrhoid ointment, whose main active ingredients were triamcinolone acetonide, lidocaine, and pentosan polysulfate sodium (PPS). The cases illustrate both the potentially damaging effect of an over-the-counter product and the need to ask patients about the topical treatments they might be using when they consult us about perianal ulcers.
Differential Diagnosis of Perianal Ulcers
| Infectious diseases | Herpes simplex, syphilis, lymphogranuloma venereum, cytomegalovirus, human immunodeficiency virus, cankers, tuberculosis, Mycobacterium avium, Entamoeba histolytica, and deep mycoses |
| Inflammatory diseases | Crohn disease, ulcerative colitis, pyoderma gangrenosum, Behcet disease, and sarcoidosis |
| Malignancy | Extramammary Paget disease, squamous cell carcinoma, Kaposi sarcoma, and skin metastasis |
| Pharmaceutical products | Nicorandil, vasoconstrictor-containing suppositories, and topical corticosteroids |
| Other | Radiotherapy, pressure ulcers, and factitious disorders |
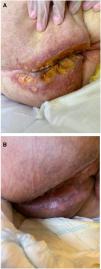
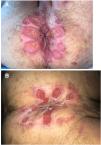
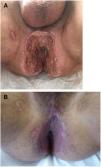
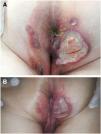
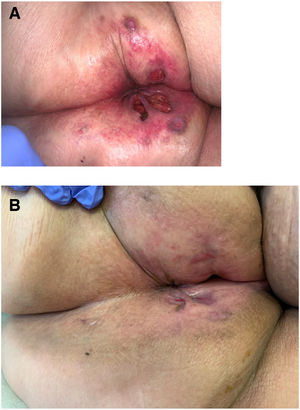
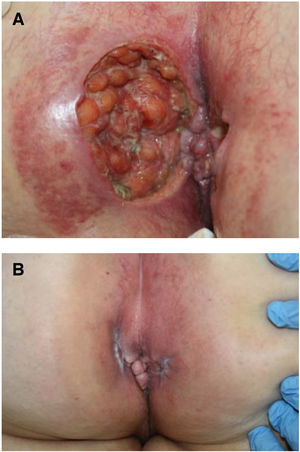
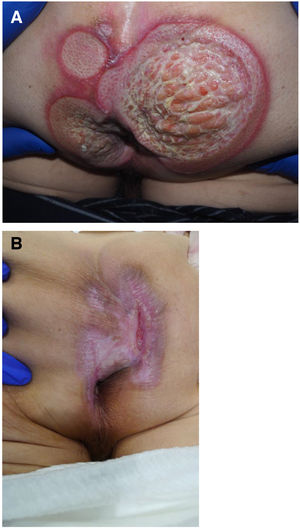
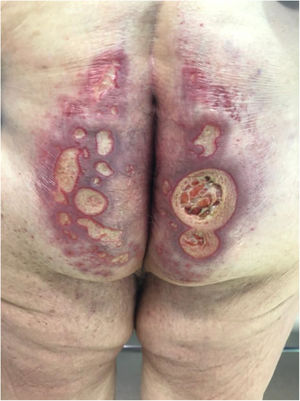
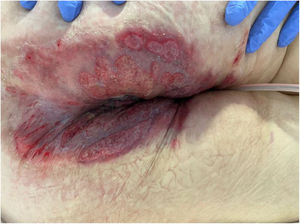
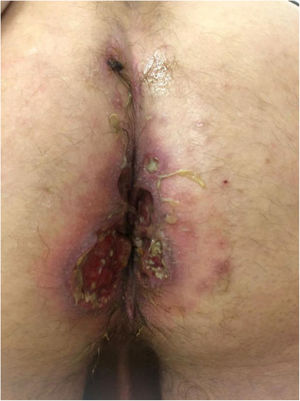
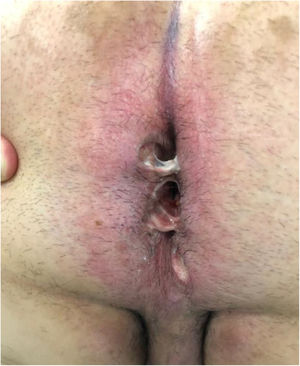
We collected 11 cases of perianal ulcer formation related to the same hemorrhoid ointment that were treated in 10 Spanish hospitals from March to October 2020. The lesions had first appeared earlier in 1 case, however. Epidemiologic, clinical, and therapeutic variables were studied for each case, and lesion progression was followed. Other causes for the perianal ulcers were ruled out.
ResultsThe 11 cases of perianal ulcers were treated with the same hemorrhoid ointment containing 3 active ingredients: 0.01% triamcinolone acetonide, 2% lidocaine, and 1% PPS. Seven of the patients were men, and 4 were women. The average age was 62 years. The ointment had been used for periods ranging from 3 weeks to 5 years (median, 6 months), although in some cases it was difficult to determine exactly how long the ointment had been applied. The main reason these patients chose to use the product was a history of hemorrhoids and discomfort. Two patients described fecal incontinence and chronic diarrhea. Overweight and/or a sedentary lifestyle was a distinctive characteristic of 10 of the patients, but none were specifically at risk for developing pressure ulcers. After physical examination and analysis of other aspects of the patients’ medical histories, we were unable to identify any alternative or concomitant condition that could explain the ulcers.
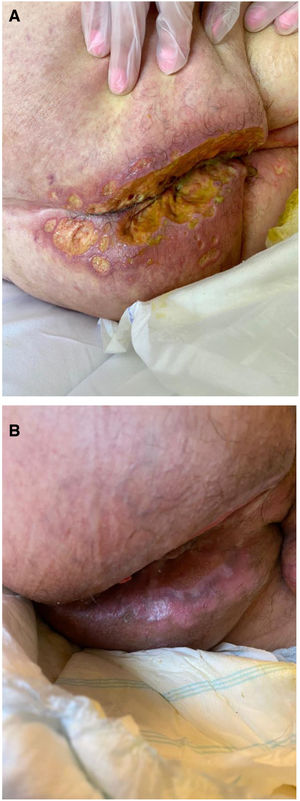
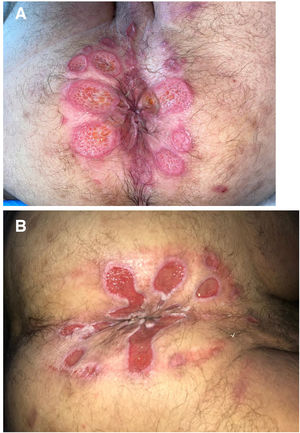
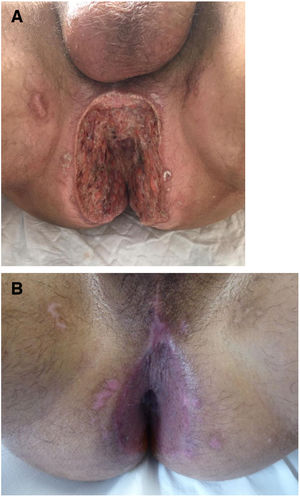
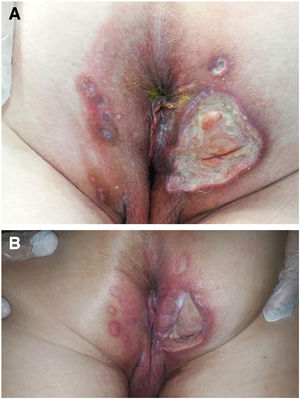
Seven patients reported complete resolution of their ulcers within 8 weeks, on average, after discontinuing use of the ointment. One patient required skin grafts to achieve complete reepithelialization. In the others, the ulcers completely disappeared on applying local measures such as dressings, irrigations, and/or topical antibiotics. All patients were prescribed systemic or topical antibiotics, given the possibility of a superinfection. Patient 8 achieved nearly complete resolution. Patients 9 and 10 were lost to follow-up, and patient 11 is still being followed but is progressing toward a complete cure. The initial appearance of the perianal ulcers in the 11 cases and their respective outcomes are shown in Figs. 1–11. The most important characteristics are summarized in Table 2.
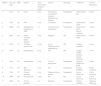
Summary of the Main Features of 11 Cases
| Patient | Sex and Age | BMI | History | Time Hemorrhoid Ointment Used | Culture | Histology | Treatment | Time to Resolution |
|---|---|---|---|---|---|---|---|---|
| 1 | F/67 | 27 | None | 2 mo | Escherichia coli, Proteus mirabilis, Enterococcus faecalis | Nonspecific | Hydrocolloid gel | 8 wks |
| 2 | F/53 | 28 | PPC | 4 mo | P mirabilis | Nonspecific | Hydrocolloid patch | 6 wks |
| 3 | M/80 | 26 | Dyslipidemia, hypertension, DM2 | 5 y | Pseudomonas aeruginosa | Nonspecific | Hydrocolloid patch, topical antibiotic | 12 wks |
| 4 | M/81 | 40 | COPD, coronary artery disease | 4 mo | Negative | NR | Hydrocolloid patch | 8 wks |
| 5 | M/30 | 23 | Cirrhosis, cocaine use | 18 mo | E coli, Staphylococcus haemolyticus | NR | Topical antibiotic | 6 wks |
| 6 | M/61 | 27 | Dyslipidemia | 6 mo | NR | NR | Copper sulfate solution, topical antibiotic | 9 wks |
| 7 | M/43 | 22 | Hemorrhoids | 4 mo | E coli, P aeruginosa | Nonspecific | Washes, partial skin grafts | 8 wks |
| 8 | F/74 | 29 | Mild dementia | 6 mo | NR | Nonspecific | Topical antibiotic | Partial resolution |
| 9 | F/83 | 27 | Chronic diarrhea | 8 mo | Staphylococcus aureus | Nonspecific | Lost to follow-up | Lost to follow-up |
| 10 | M/54 | 26 | Hemorrhoids | 3 wks | NR | NR | Lost to follow-up | Lost to follow-up |
| 11 | M/55 | 29 | Chronic diarrhea | 3 y | Negative | Nonspecific | Topical antibiotic | Ongoing follow-up |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; DM2, type 2 diabetes mellitus; F, female; M, male; NR, not requested; PPC, panproctocolectomy.
Perianal ulcers have been associated with multiple causes, but the possibility that the culprit might be a topical medication should always be considered. Few reports of an association between these ulcers and a pharmaceutical product have been published, however. In fact, this is the first case series to document a link to a hemorrhoid ointment. These 11 patients, treated in 10 Spanish hospitals, developed perianal ulcers after using the same ointment, and their ulcers cleared after they discontinued its use.
The active ingredients involved were 0.01% triamcinolone acetonide, 2% lidocaine, and 1% PPS. The ingredient most likely to cause skin damage would probably be triamcinolone acetonide, a corticosteroid of intermediate potency. Corticosteroids are well known for their vasoconstriction and the risk of skin atrophy with continued use. Cases of ulceration in intertriginous areas, including the perianal region, also exist. Another observation that allows us to hypothesize that the corticosteroid played a role in the development of these ulcers is the atrophied appearance of the skin in the region and the presence of telangiectasia around some of the lesions that resolved within a few weeks of discontinuing application of the ointment.
Lidocaine is a local anesthetic that acts as either a vasoconstrictor or vasodilator depending on the concentration used. It is theoretically possible that lidocaine might enhance the vasoconstrictive effects of a corticosteroid, but we think it unlikely that any synergy between the 2 ingredients would be great. On the other hand, the anesthetic effect of this drug could desensitize the perianal region, introducing a neuropathic-like processing component that might favor the formation of pressure ulcers. Nevertheless, we note that none of the patients in our series had reduced mobility or were at risk for this type of ulcer.
PPS, a low-molecular weight heparinoid with an anticoagulant fibrinolytic action, can induce thrombocytopenia and thrombosis.5 Although skin necrosis due to low-molecular-weight heparins can develop, usually locally at the site of injection,6 the pathophysiology of this phenomenon is still poorly understood, and no reports indicate that PPS can cause necrosis. Furthermore, none of the biopsy specimens from the patients in our series showed evidence of microthrombi.
Of great importance are the shared clinical features of patients in this series and the common site where all of them applied the ointment. The perianal region offers ideal conditions of occlusion, humidity, and risk of superinfection; these conditions combine to favor the formation and growth of ulcers. In addition, this region tends to have poor perfusion, as shown by cases of perianal ulcers related the use of nicorandil or the insertion of vasoconstrictor-containing suppositories.
We think that a noteworthy demographic characteristic of the patients in this series is a low social and educational level that could account for inappropriately persistent application of the hemorrhoid ointment and the difficulty some had in completely discontinuing its use. Also relevant is overweight and/or a sedentary lifestyle, characteristics shared by nearly all the patients. This finding probably contributed to the underlying pathophysiology of ulceration. Therefore, the combination of the anatomical site involved, a certain patient profile, and improper use of a potentially injurious ointment not only contributed to the formation of the ulcers but also to their marked growth.
We observed complete resolution of lesions in 7 patients after they discontinued the hemorrhoid ointment and applied physical methods to promote reepithelialization. None of the clinical features, laboratory tests, or histologic findings for these patients were consistent with any diagnosis other than perianal ulcers. The rapid improvement of some of the long-established ulcers after the ointment was discontinued provided solid evidence that this product had caused the lesions.
Finally, considering that the hemorrhoid ointment in question has been on the market for over 40 years, it remains to be determined why it is suddenly causing ulcers now. A possible reason might be more widespread use as a result of greater availability through various types of commercial outlets. Another salient aspect of the context for these recent cases is the current COVID-19 pandemic and consequent lockdown conditions in Spain, which have undoubtedly changed behaviors and lifestyles, possibly aggravating sedentary habits previously in place. However, not all of the patients reported that the onset of ulceration occurred during the pandemic months. We take this opportunity to mention that most of the cases were reported to the Spanish Agency for Medicines and Health Products (AGEMED) to alert the authorities to this growing and previously unknown adverse event.
To conclude, we have presented 11 previously unreported cases of perianal ulcers related to use of the same hemorrhoid ointment. Our purpose has been to warn of this adverse event, reporting what we consider to be a public health threat given that the hemorrhoid ointment continues to be sold and used without medical prescription or supervision. We are also aware of the limitations of this study and the need for future research to more clearly discover the true magnitude of the problem we present here.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The case comparisons in this article were possible thanks to the virtual forum Dermachat, on which 600 dermatologists discuss cases.
Please cite this article as: Marín-Piñero D, Iglesias-Sancho M, Company-Quiroga J, Martínez-Moran C, Perez-Feal P, Vazquez-Osorio I, et al. Múltiples úlceras perianales en relación con el uso de una pomada antihemorroidal con acetónido de triamcinolona, lidocaína y pentosano polisulfato sódico como principios activos: una serie de 11 pacientes españoles. Actas Dermosifiliogr. 2021;112:654–660.