Malignant melanoma is among the malignant tumors whose incidence has risen markedly in recent decades. For many years the medical community debated the potential adverse effects of female hormones (whether of exogenous or pregnancy-related endogenous origin), on melanocytic nevi and malignant melanoma. Given that women have been delaying pregnancy until their thirties or forties and that the incidence of malignant melanoma increases in those decades, the likelihood of this tumor developing during pregnancy has increased. Recent clinical and experimental evidence has suggested that pregnancy does not affect prognosis in malignant melanoma and that it does not seem to lead to significant changes in nevi. This review examines the relationship between malignant melanoma and hormonal and reproductive factors. Evidence was located by MEDLINE search (in PubMed and Ovid) for articles in English and Spanish for the period from 1966 to March 2010; additional sources were found through the reference lists of the identified articles.
El melanoma maligno (MM) es uno de los tumores malignos que más ha aumentado su incidencia en las últimas décadas. Durante muchos años hubo controversia en la comunidad médica en relación con el efecto potencialmente adverso de las hormonas femeninas (exógenas o endógenas asociadas al embarazo) sobre los nevus melanocíticos y el MM. Considerando que las mujeres han retrasado la maternidad hasta la tercera y cuarta décadas de la vida, y que la incidencia de MM aumenta en estas décadas, la probabilidad de aparición de un MM durante el embarazo es mayor.
Evidencias clínicas y experimentales recientes permiten sugerir que el embarazo no influye en el pronóstico del MM, y que no parece causar cambios significativos en los nevus. La finalidad de este artículo es revisar la asociación entre MM, nevus y factores hormonales y reproductivos.
Se realizó una búsqueda de artículos utilizando la base bibliográfica Medline y los buscadores “Pubmed” y “Ovid”, en inglés y español, en el periodo de 1966 a marzo del 2010. Los artículos fueron revisados y se consiguieron referencias adicionales de las bibliografías.
The influence of pregnancy on melanocytic nevi and malignant melanoma has been debated for years. In clinical practice, it is not uncommon to examine pregnant women alarmed by morphologic changes in one or more nevi.1–3
Considering that women have been delaying pregnancy until their thirties, forties, or even fifties, and that the incidence of malignant melanoma increases in these decades of life, the likelihood of this type of cancer being diagnosed during pregnancy has increased, as has the number of women who want to become pregnant or use oral contraceptives to avoid becoming pregnant after a diagnosis of malignant melanoma.4,5
Malignant melanoma in women is rare before puberty but its incidence increases during childbearing years (up to the fifth decade) and reduces after menopause.6 Certain changes in pigmentation, such as melasma, are associated with pregnancy, oral contraceptive use, and hormone replacement therapy (HRT). The recent finding that the estrogen receptor β (ER-β) is expressed in benign nevi, dysplastic nevi, malignant lentigos, and malignant melanomas of different thicknesses7–10 has led to speculation about the relationship between hormones, nevi, and malignant melanoma.11
Melanocytic Nevi and PregnancyFew studies have objectively evaluated changes in melanocytic nevi during pregnancy.
Commonly noted subjective changes include an increase in size or a darkening of pigmented skin lesions. These self-perceived changes have been reported by between 10.5%1 and 32.5%12 of pregnant women, and affect lesions on the breasts and abdomen (areas that expand during pregnancy) and nonmelanocytic skin lesions such as dermatofibromas and acrochordons.
The first 2 prospective studies to report changes in melanocytic nevi during pregnancy were published in the 1990s. In 1991, Ellis13 described objective nevus changes in 17 pregnant patients with dysplastic nevus syndrome during 22 pregnancies. The patients had melanocytic nevi with atypical morphology (diameter > 5mm, dyschromia, and irregular, poorly defined borders) and histologic findings including cellular and architectural atypia. The changes were documented photographically. The author concluded that the rate of clinical nevus change was 3.9 times higher in pregnant women than in nonpregnant women. This observation is consistent with the nature of dysplastic nevus syndrome, as atypical nevi are more prone to change, and patients with this syndrome have a higher risk of developing melanoma.
In 1997, Pennoyer et al14 evaluated changes in the size of 129 nevi in 22 white women, all of whom were pregnant. They took photographs of all nevi measuring at least 2mm that were located on the back in the first and third trimester of pregnancy. The back was chosen because other areas of the body, such as the abdomen and breasts, expand during pregnancy and possibly cause changes in nevi. Of the 129 nevi analyzed, only 8 (6.2%) changed in diameter, with 4 increasing by 1mm and 4 decreasing by 1mm. According to the authors, despite the small sample included in their study, the results suggest that pregnancy is not associated with significant changes in the size of nevi on the back.
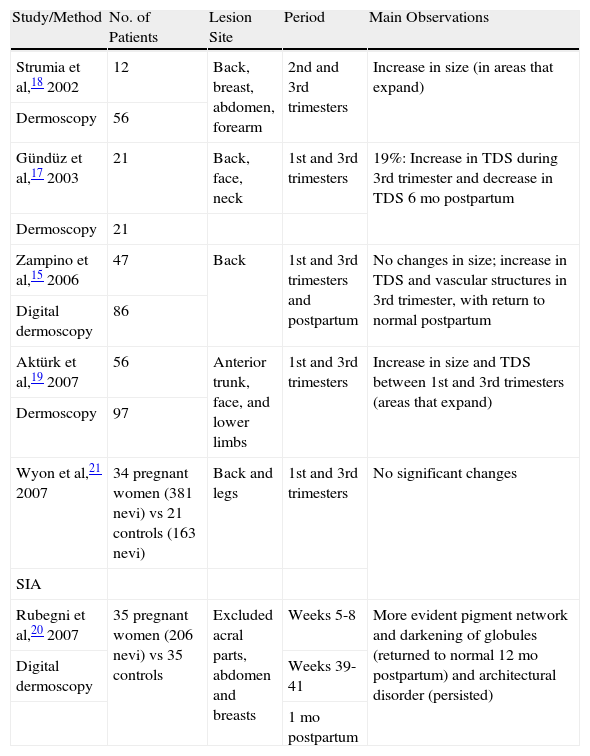
The first prospective studies of melanocytic nevi during pregnancy to use dermoscopy appeared in the early 2000s (Table 1).
Prospective Studies With Objective Evaluation of Melanocytic Nevi During Pregnancy.
| Study/Method | No. of Patients | Lesion Site | Period | Main Observations |
| Strumia et al,18 2002 | 12 | Back, breast, abdomen, forearm | 2nd and 3rd trimesters | Increase in size (in areas that expand) |
| Dermoscopy | 56 | |||
| Gündüz et al,17 2003 | 21 | Back, face, neck | 1st and 3rd trimesters | 19%: Increase in TDS during 3rd trimester and decrease in TDS 6 mo postpartum |
| Dermoscopy | 21 | |||
| Zampino et al,15 2006 | 47 | Back | 1st and 3rd trimesters and postpartum | No changes in size; increase in TDS and vascular structures in 3rd trimester, with return to normal postpartum |
| Digital dermoscopy | 86 | |||
| Aktürk et al,19 2007 | 56 | Anterior trunk, face, and lower limbs | 1st and 3rd trimesters | Increase in size and TDS between 1st and 3rd trimesters (areas that expand) |
| Dermoscopy | 97 | |||
| Wyon et al,21 2007 | 34 pregnant women (381 nevi) vs 21 controls (163 nevi) | Back and legs | 1st and 3rd trimesters | No significant changes |
| SIA | ||||
| Rubegni et al,20 2007 | 35 pregnant women (206 nevi) vs 35 controls | Excluded acral parts, abdomen and breasts | Weeks 5-8 | More evident pigment network and darkening of globules (returned to normal 12 mo postpartum) and architectural disorder (persisted) |
| Digital dermoscopy | Weeks 39-41 | |||
| 1 mo postpartum |
Abbreviations: SIA, spectrophotometric intracutaneous analysis; TDS, total dermoscopy score.
In 2006, Zampino et al15 evaluated dermoscopic changes in melanocytic nevi during and after pregnancy. They analyzed 86 nevi located on the back of 47 pregnant women using digital dermoscopy. The nevi were evaluated 3 times: during the first trimester, during the third trimester, and 6 months postpartum. The dermoscopic changes detected were classified as follows: a) no significant changes in size (eg, small increase during pregnancy in areas prone to greater skin expansion but a return to normal postpartum); b) a decrease in overall pigmentation and the presence of a less prominent pigment network during pregnancy and particularly 6 months after delivery, possibly explained by the fact that the participants reported less sun exposure during this period; c) an increase in the number of vascular structures (dotted vessels and to a lesser degree comma-shaped vessels) during pregnancy, with a return to normal postpartum (these changes were considered to be physiological alterations induced by pregnancy hormones); and d) an increase in total dermoscopy score, calculated according to the ABCD rule proposed by Stolz et al.16 The increase in total dermoscopy score and changes in symmetry during pregnancy indicate that hormones exert an intrinsic influence on melanocytic activity, leading to a slightly more irregular dermoscopic appearance that returns to normal postpartum. The above findings are consistent with the observations of Gündüz et al.17
Strumia et al18 evaluated the dermoscopic features of 56 nevi with a diameter of over 4mm in 12 pregnant women examined during the second and third trimester. In areas prone to expansion during pregnancy (eg, the breasts and the abdomen), pigmented skin lesions with a reticular pattern grew in size but did not change shape, while those with a globular pattern presented an increased number of peripheral brown globules. No significant changes were noted in the size or color of nevi in areas that change only slightly during pregnancy (eg, the back).
Aktürk et al,19 also using dermoscopy, analyzed 97 nevi in 56 pregnant women during the first and third trimester, and found a statistically significant increase in both size and total dermoscopy score; most of the nevi that grew were on the anterior surface of the trunk. The authors also observed new dot formation in 6 lesions and the appearance of new nevi in 3 women in the third trimester. Their results were similar to those reported by Gündüz et al17 and Strumia.18
Rubegni et al20 used digital dermoscopy to evaluate nevi in 35 pregnant women and an equal number of controls. They analyzed all nevi larger than 4mm before pregnancy and excluded pigmented skin lesions in acral parts and on the abdomen and breasts; they also excluded ephelides and lesions with clinical or dermoscopic atypia. They studied a total of 206 nevi between weeks 5 and 8 and between weeks 39 and 41 of pregnancy, as well as 12 months after delivery. They noted a thicker and more evident pigment network, a darkening of globules (lesions with a globular pattern), and architectural disorder. The pigment network and globules had returned to normal by 12 months postpartum but the architectural disorder persisted.
In a recent study, Wyon et al21 evaluated changes in melanocytic nevi during pregnancy using spectrophotometric intracutaneous analysis. This method uses light between the visible and the near-infrared region, allowing deeper penetration into the skin and subsequent computerized analysis of images obtained. The authors compared images of 384 melanocytic nevi from 34 women in the first and third trimester of pregnancy with those of 163 melanocytic nevi from a control group of nonpregnant women. They concluded that there were no significant differences between the 2 groups.
In summary, melanocytic nevi can undergo reversible changes during pregnancy. These include darkening, a progressive reduction in thickness, the appearance of a prominent reticular pattern or dots or globules, and an increase in vascularization and size (especially in areas prone to greater expansion such as the anterior surface of the trunk).15,17,19,20,22,23 Nonetheless, any lesions that present changes suggestive of malignant transformation (atypical pigmented network, blue-whitish veil, atypical vascular pattern, etc.) should be biopsied immediately, just as would be done in any patient. Nevi in patients with dysplastic nevus syndrome should be analyzed by digital dermoscopy at the beginning of pregnancy and at 3-month intervals to screen for possible changes.2
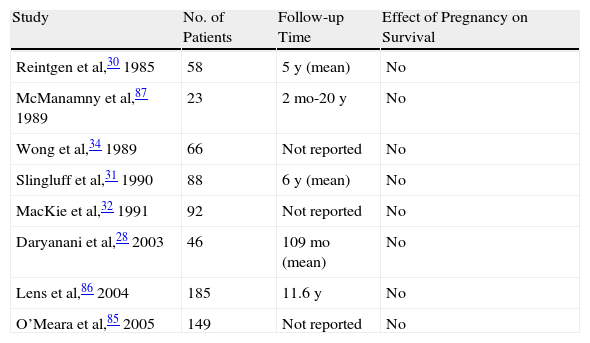
Melanoma and Hormonal and Reproductive FactorsMalignant Melanoma Diagnosed During PregnancyAlthough results obtained over the course of many years of investigation initially suggested that pregnancy might increase the risk of melanoma,24–27 current clinical evidence does not support this hypothesis. Numerous studies, such as that of Daryanani et al,28 have provided evidence that the clinical course of disease, together with prognosis and overall survival, in pregnant women with localized malignant melanoma (American Joint Committee on Cancer [AJCC] stages I-II) is similar to that of nonpregnant women (Table 2). In the case of a pregnant woman with malignant melanoma, prognosis depends primarily on tumor thickness and on the presence or absence of ulceration.
Case-Control Studies of Localized Melanoma Diagnosed During Pregnancy.
| Study | No. of Patients | Follow-up Time | Effect of Pregnancy on Survival |
| Reintgen et al,30 1985 | 58 | 5 y (mean) | No |
| McManamny et al,87 1989 | 23 | 2 mo-20 y | No |
| Wong et al,34 1989 | 66 | Not reported | No |
| Slingluff et al,31 1990 | 88 | 6 y (mean) | No |
| MacKie et al,32 1991 | 92 | Not reported | No |
| Daryanani et al,28 2003 | 46 | 109 mo (mean) | No |
| Lens et al,86 2004 | 185 | 11.6 y | No |
| O’Meara et al,85 2005 | 149 | Not reported | No |
In a pooled analysis, Karagas et al29 reviewed 10 case-control studies (total of 5590 women [2391 cases and 3199 controls]) to evaluate the effect of pregnancy on the risk of developing malignant melanoma, but found no association. In another 2 studies, however, Reintgen et al30 and Slinguff et al31 found significantly shorter disease-free survival in pregnant women compared to nonpregnant women, and they also reported that lymph node metastasis was the most common form of recurrence.
Several studies have shown an increase in tumor thickness in pregnant women compared to nonpregnant women, probably due to delayed diagnosis.30–33 Early diagnosis is very important and suspicious lesions should be biopsied immediately, without waiting until the end of the pregnancy.
Several well-controlled trials and studies involving large numbers of patients28,30,32,34 have found no differences between women diagnosed with malignant melanoma during pregnancy and controls in terms of primary lesion site or histologic type.
Evaluation and Treatment of Malignant Melanoma Diagnosed During PregnancyIn general, the evaluation of malignant melanoma is similar in pregnant and nonpregnant women, with treatment depending on disease stage. Nonetheless, certain measures must be taken during a pregnancy to protect the fetus.
Localized melanomas diagnosed during pregnancy must be excised using local anesthesia and wide margins based on current recommendations.32,35 Lidocaine is considered to be a safe local anesthetic for use during pregnancy29 but general anesthesia should be avoided.
If the tumor is associated with a high risk of recurrence, a sentinel lymph node (SLN) biopsy may be considered for disease-staging purposes.36,37 The safety of this procedure in pregnancy is a matter of debate. Consent must be obtained from the patient following discussion of the potential risks, benefits, and limitations of the procedure. Patients must be informed that SLN biopsy has not been proven to increase overall survival and therefore may not be necessary if the patient does not want treatment in the future. The safest method for performing SLN biopsy in pregnant women has not been established, with some medical centers and surgeons using a radiotracer (eg, technetium Tc 99m sulfur-colloid), others using blue dye, and others using a combination of the two.36 Although the risk for the mother and the fetus is relatively small, the procedure does carry certain risks such as allergic reactions to the dye and fetal exposure to radiation. Some doctors prefer to avoid using radiotracers during pregnancy38 while others believe that the radiation dose to which the fetus is exposed is below the teratogenic threshold.4,36 Schwartz et al4 showed the use of blue dye in pregnancy to be associated with a risk of anaphylaxis of between 0.7% and 1.1%. To avoid this risk, radiotracers can be used in isolation. The fetus is exposed to a radiation dose of under 5 mGy during SLN biopsy. (The Nuclear Medicine Society recommends performing a pregnancy test when a patient has to undergo any procedure in which the fetus would be exposed to a radiation dose of over 50 mGy.) In the case of a pregnant woman in her second or third trimester, it would be reasonable to postpone the biopsy until after delivery if the malignant melanoma has been excised with wide margins and if the patient is monitored regularly.
The safety of imaging techniques in pregnant women to screen for distant metastasis is also a matter of debate. Chest radiography with radiation protection can be performed safely, as can abdominal ultrasound imaging.39 Computed tomography (CT) with intravenous contrast and positron emission tomography are generally contraindicated as they emit high doses of radiation, which would be absorbed by the fetus.40 Magnetic resonance imaging is safer than CT imaging, but it is not recommended during the first trimester of pregnancy because the radiofrequency it employs heats tissues and exposes the fetus to excessive noise, which can cause high-frequency hearing loss in neonates.41
There is very limited experience with the use of chemotherapy or interferon to treat advanced metastatic disease in pregnant women due to the limited effectiveness of these methods and the potential adverse effects for the fetus.42 In our review of the literature, we found 36 reports of the use of dacarbazine in pregnant women, 8 of whom were treated in the first trimester43–47 and 28 of whom were treated during the second or third trimesters.47–52 In the first group, there were 2 cases of congenital abnormalities (microphthalmia and severe hyperopia),44 while in the second group, there was 1 fetal death47 and 1 case of syndactyly.50 In all 4 cases, dacarbazine had been administered in combination with other cytotoxic drugs. Nonetheless, the risk of secondary malignancy might be underestimated as the follow-up period for neonates exposed to chemotherapy in utero was very short (mean of 14 months). One report from Japan describes how a woman with stage III malignant melanoma treated with DAV-feron (dacarbazine, nimustine, vincristine, and interferon-beta) during the second trimester of pregnancy gave birth to a healthy baby at 35 weeks, with no signs of placental involvement.53
Radiotherapy during pregnancy can cause fetal malformations, mental retardation, and even death, and it is poorly effective in malignant melanoma. There has been 1 report of the use of radiotherapy in 2 patients with malignant melanoma and symptomatic brain metastases, with no impact on the neonate.54
Risk for the FetusIt has been estimated that malignant melanoma causes complications in 0.1 to 2.8 of every 1000 pregnancies. In a review of the literature, Alexander et al55 found that placental and fetal metastasis was very rare in cancer in general, with only 87 cases reported in the last 140 years. Although malignant melanoma is not the most common tumor in pregnancy, it probably carries the greatest risk of placental or fetal metastasis as it accounted for 27 (31%) of the 87 cases reported.
The prognosis for the fetus in a pregnant woman with malignant melanoma depends on maternal stage of disease. Prognosis is generally excellent, except in cases of disseminated disease. Transplacental transmission of malignant melanoma to the fetus is very rare and has only been described in women with distant metastasis. It has been estimated that the fetus will be affected in just 25% of cases in which there is placental involvement. Prematurity is a common complication in children born to mothers with placental disease; a mean gestational age of 34 weeks has been reported, but with low mortality attributable to premature birth.55
When a fetus is affected by malignant melanoma metastasis, the most common sites affected are the skin and the liver; these metastases cause intrauterine or postpartum death in over 80% of cases.55,56
A thorough macroscopic and histologic investigation of the placenta of women with advanced disease is recommended to detect possible metastasis. This investigation should include immunohistochemical studies (S-100, HMB-45, Melan-A, MART-1).55 Periodic follow-up for at least 24 months is recommended in the case of children without metastasis born to women with confirmed placental involvement. Tests should include a baseline chest radiograph, liver function tests, lactate dehydrogenase testing, and a thorough skin examination.
Due to the rarity of congenital and infantile malignant melanoma, there are no clinical guidelines on how to treat this disease in children born to mothers with placental metastasis. There are also no publications on adjuvant treatment with high doses of interferon alpha in these children.55
Malignant Melanoma Diagnosed Before PregnancySeveral studies performed in small series of patients have concluded that the prognosis of women who become pregnant after being diagnosed with localized malignant melanoma does not change.30,32 Advice on future pregnancy varies from one specialist to the next, with some recommending not becoming pregnant. A considerable proportion of patients with localized malignant melanoma (AJCC stages I-II) may experience recurrence. While approximately 50% of recurrences in patients with thick lesions occur within 3 years, they may occur later in some cases (up to 10 years after diagnosis), even in patients with thin lesions.4
The time a woman who has had malignant melanoma should be advised to wait before becoming pregnant varies from case to case, and primarily depends on the risk of recurrence (tumor thickness and ulceration), the age of the patient, and the patient's desire to become pregnant.4 This time ranges between 0 and 5 years. For example, a 40-year-old woman with a malignant melanoma of 0.3mm thickness would not be advised to wait if she wanted to become pregnant and considered the risk (1% to 3% for 5-year recurrence) acceptable. In contrast, a 21-year-old woman with a 4.0-mm malignant melanoma would be advised to wait for 3 to 5 years (period with the greatest risk of recurrence).
Individual case management is recommended in all cases and all possible risks should be discussed in order for the patient to take an informed decision.
Malignant Melanoma Diagnosed After PregnancyPrevious pregnancy does not appear to influence the survival of women diagnosed with localized malignant melanoma.25 It has been suggested, however, that women who have had multiple pregnancies (5 or more) before a diagnosis of malignant melanoma have a survival advantage,57–59 but few studies have investigated this.
Use of Oral Contraceptives or Hormone Replacement Therapy and Risk of Developing Malignant MelanomaThe association between oral contraceptive use and the risk of developing malignant melanoma has been discussed in several studies, with inconsistent findings.
Various epidemiological studies29,60–81 have shown that the use of oral contraceptives does not increase the risk of malignant melanoma.
In their pooled analysis of 5590 women from 10 studies, Karagas et al29 did not find an increased risk of malignant melanoma among oral contraceptive users. Meanwhile, there is no specific information on whether or not the use of oral contraceptives increases the risk of recurrence in women with an established diagnosis of malignant melanoma.
Although few studies have investigated the association between HRT and malignant melanoma,60,62,65,68,69,72,77,82 to date no epidemiological evidence has shown an increased risk of malignant melanoma in women on HRT. The use of oral contraceptives or HRT is not contraindicated in patients with a thin lesion and a low risk of recurrence.5,83,84
As far as clinical and experimental evidence is concerned, no clear association has been shown between hormones, nevi, and malignant melanoma. Indeed most of the clinical evidence suggests that there is no association: it has not been clearly established that nevi undergo significant physiological changes during pregnancy; controlled studies have shown that pregnancy does not affect prognosis in malignant melanoma; and epidemiological studies have shown no effect for exogenous hormones (contraceptives and HRT) on the risk of malignant melanoma.5,88,89 Of interest are the recent findings that ER-β is the predominant estrogen receptor in melanocytic lesions7–10 and that it is strongly expressed in severely dysplastic nevi and malignant lentigos compared to thick malignant melanomas. Further studies, however, are required in this area to determine the relevance of these findings.
ConclusionsAlthough slight changes can occur in the diameter and dermoscopic features of nevi during pregnancy, above all in areas prone to greater expansion such as the anterior surface of the trunk, the nevi concerned generally return to normal postpartum. Nevertheless, any lesions that present features suggestive of possible transformation to malignant melanoma (atypical pigmented network, blue-whitish veil, atypical vascular pattern, etc.) should be biopsied immediately, just as would be done in any patient.
Women with dysplastic nevus syndrome experience more clinical changes in nevi when they are pregnant.
More prospective studies on changes in nevi during pregnancy are needed, and these should preferably involve clinical examination, photographs, and digital dermoscopic images.
Based on the results of a small number of well-controlled studies, prognosis in malignant melanoma is the same whether a women is diagnosed with the disease before, during, or after pregnancy. No significant differences in 5-year-survival have been demonstrated between pregnant and nonpregnant women, and hence therapeutic abortion is not recommended as an option for increasing survival.
The prognosis for a fetus in a woman with malignant melanoma depends on the stage of maternal disease. Fetal metastases are rare and may only occur in women with widely disseminated disease. It has been estimated that fetal metastasis occurs in just 25% of cases of placental involvement.
The treatment of a thin malignant melanoma—excision with wide surgical margins, local anesthetic, and close clinical monitoring—is the same in pregnant and nonpregnant patients. Nonetheless, in the case of pregnant patients who are candidates for SLN biopsy or imaging studies for assessing disease stage or screening for distant metastases, the approach varies enormously from one center to the next and decisions should be taken on a case-by-case basis.
The recommended time a woman should wait before becoming pregnant after a malignant melanoma varies from one patient to the next and depends primarily on the risk of recurrence (tumor thickness, ulceration, SLN metastasis), age of the patient, and the patient's desire to become pregnant. There is no one answer and patients should be well informed about the risks associated with their disease in order to taken an informed decision. Women with high-risk malignant melanoma and an increased risk of recurrence should be advised to wait for 3 to 5 years before becoming pregnant.
Oral contraceptive use does not increase the risk of malignant melanoma in women. Meanwhile, there is no specific information on whether or not the use of oral contraceptives increases the risk of recurrence in women with an established diagnosis of malignant melanoma.
Although few studies have been performed in this area, HRT does not appear to increase the risk of developing malignant melanoma, but it is not known whether or not it influences the risk of recurrence. The use of oral contraceptives or HRT is not contraindicated in patients with thin malignant melanomas in the absence of reasonable alternatives.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Borges V, et al. Nevus, melanoma y embarazo. Actas Dermosifiliograr. 2011;102:650-657.