Low-dose rituximab is a protocol used in several autoimmune diseases, that has also shown to be effective and safe in pemphigus vulgaris.
ObjectivesTo study whether low-dose rituximab is also effective for bullous pemphigoid.
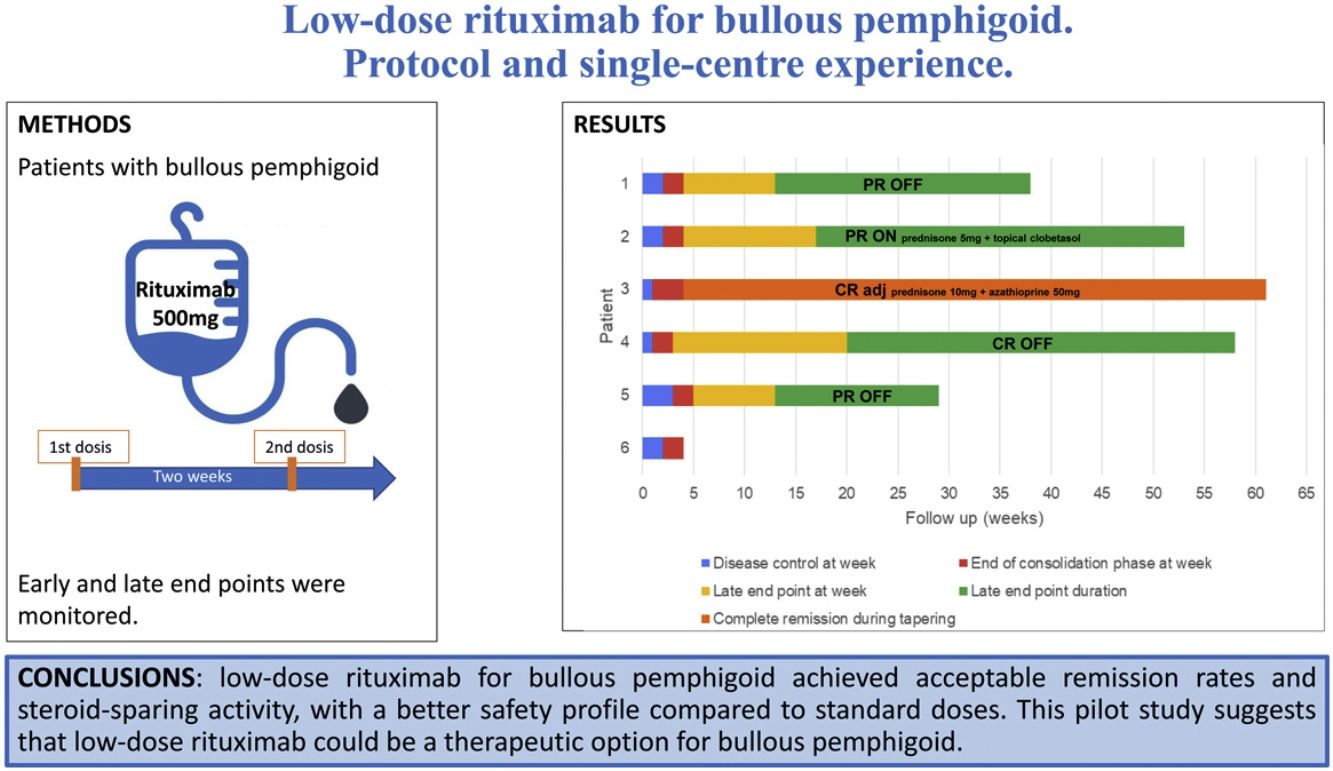
MethodsPatients with BP were treated with a single cycle of two infusions of rituximab 500mg at an interval of 2 weeks. Early and late end points were monitored.
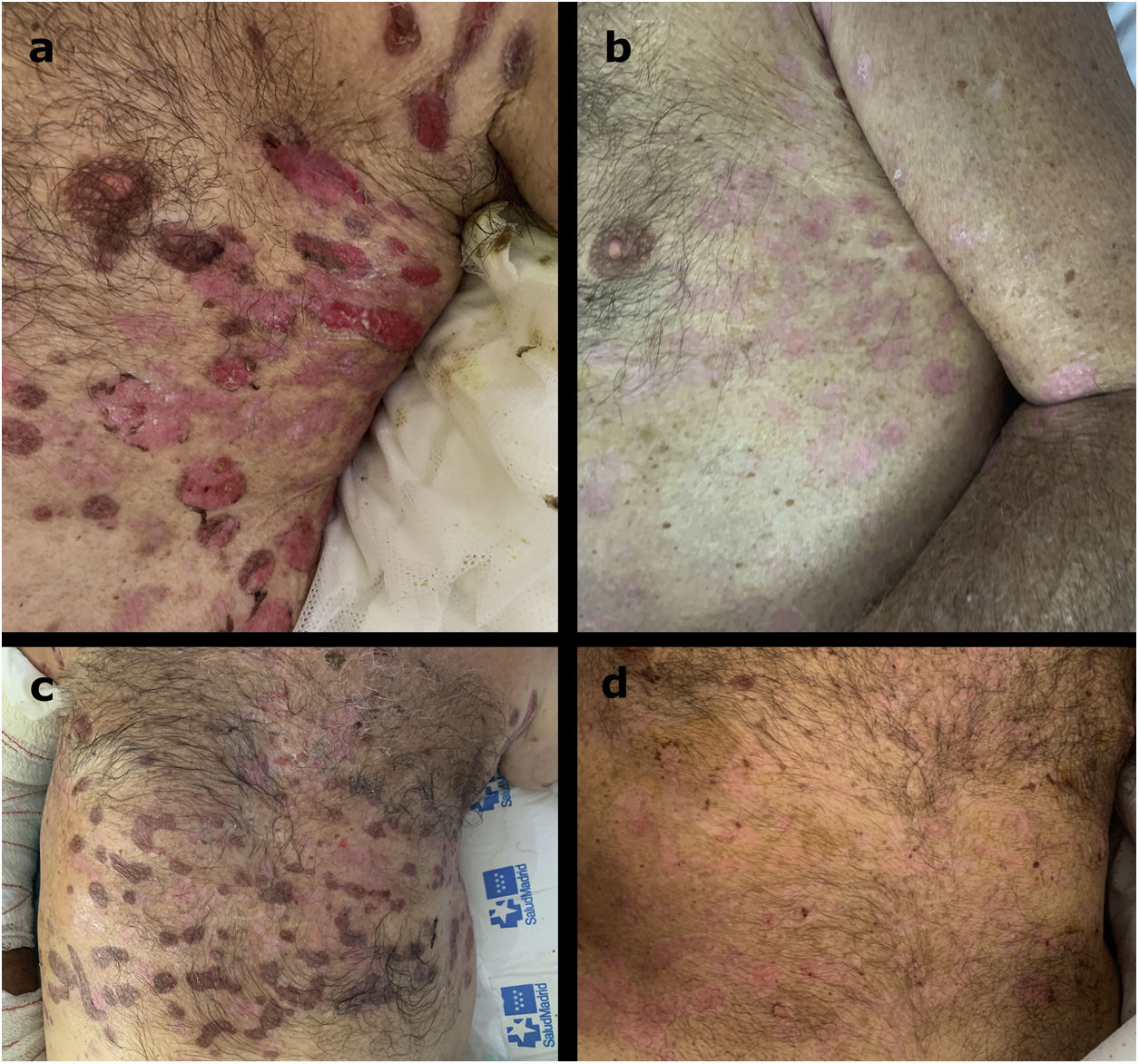
ResultsSix patients, five males and a female, with a mean age of 78.6 years (range 65–89) and a mean history of BP of 6.7 months (range 2–16) were included. A rapid and marked response was observed after a single cycle of treatment, with a mean time to disease control and to end of consolidation phase of 1.9 (range 1–3), and 4 weeks (range 3–5), respectively. Four patients achieved a late end point at a mean of 15.75 weeks (range 13–20). Three of them achieved partial remission with no therapy (two patients) or with minimal therapy (one patient), and one of them achieved complete remission with no therapy. One patient has 6 weeks of clinical follow-up after rituximab administration. The remaining patient relapsed 4 weeks after the rituximab treatment, and remains in complete remission with more than minimal therapy. One patient had a herpetic gingivostomatitis related to rituximab.
ConclusionsLow-dose rituximab for BP achieved acceptable remission rates and steroid-sparing activity, with a better safety profile and a lower cost, compared to standard doses. This pilot study suggests that low-dose rituximab could be a therapeutic option for BP.
La administración de dosis bajas de rituximab es un protocolo utilizado en diversas enfermedades autoinmunes, que ha demostrado también su eficacia y seguridad para el pénfigo vulgar.
ObjetivosDeterminar si rituximab a dosis bajas es efectivo para el penfigoide ampolloso (PA).
MétodosSe trató a los pacientes con PA con un ciclo único de 2 infusiones de rituximab 500mg con un intervalo de 2 semanas. Se monitorizaron los puntos temprano y final tardío.
ResultadosSe incluyeron en el estudio 6 pacientes, 5 varones y una mujer, con una edad media de 78,6 años (rango: 65.89) e historia media de PA de 6,7 meses (rango: 2-16). Se observó una respuesta rápida y acusada tras un ciclo único de tratamiento, con un tiempo medio hasta el control de la enfermedad y el final de la fase de consolidación de 1,9 (rango: 1-3) y 4 semanas (rango: 3-5), respectivamente. Cuatro pacientes lograron un punto final tardío a una media de 15,75 semanas (rango: 13-20). Tres de ellos lograron una remisión parcial sin terapia (2 pacientes) o con terapia mínima (un paciente), logrando uno de ellos la remisión completa sin terapia. A un paciente se le realizó un seguimiento de 6 semanas tras la administración de rituximab. El paciente restante sufrió una recaída transcurridas 4 semanas del tratamiento de rituximab, permaneciendo en remisión completa con terapia mínima. Un paciente manifestó gingivoestomatitis herpética relacionada con rituximab.
ConclusionesLa administración de dosis bajas de rituximab para PA logró tasas de remisión aceptables y reducción de esteroides, con un mejor perfil de seguridad y un menor coste, en comparación con las dosis estándar. Este estudio piloto sugiere que la administración de bajas dosis de rituximab podría ser una opción terapéutica para el PA.
Bullous pemphigoid (BP) is the most frequent autoimmune blistering disease.1 Patients with BP have an increased morbidity and mortality resulting mainly from advanced age and underlying disorders, but also from adverse events of the immunosuppressive therapies used for this condition.1,2 Topical and oral corticosteroids are the mainstay treatment for BP.1 Second line treatment includes several non-validated therapies such as tetracyclines, azathioprine, mycophenolate, methotrexate, chlorambucil or dapsone.1,2 This challenging benefit–risk balance leads to a constant search for newer and safer therapies.
Rituximab has been used for the treatment of autoimmune bullous diseases. Standard doses approved for lymphomas (375mg/m2 weekly for 4 weeks) and rheumatoid arthritis (RA) (two infusions of 1000mg repeated in 2 weeks) are the most used protocols, and have shown to be effective in pemphigus vulgaris (PV),3,4 BP,2,5,6 and other autoimmune bullous diseases.7 A low-dose rituximab protocol of two infusions of 500mg has also been used for PV, showing a good safety profile and therapeutic response.3,4 Thus, we hypothesized this lower dose might be effective also in BP. Herein we report our experience in the treatment of BP with low-dose rituximab.
MethodsPatientsThis retrospective, single-center case series included patients with BP treated with low-dose rituximab at Hospital Universitario Ramón y Cajal (Madrid, Spain) between September 2019 and April 2020. Diagnosis of BP was established by clinical, histological and immunological criteria, according to the European guidelines.1 All patients had failure for initial control after treatment with conventional therapies for a minimum of 4 weeks, as defined by the International Pemphigoid Committee.8 Treatment with low-dose rituximab was approved by our institution as an off-label compassionate use.
Pretreatment workupPretreatment workup included a complete blood test, chest X-ray, Mantoux test, and several microbiological studies to rule out viral infections including Hepatitis B virus, Hepatitis C virus, and HIV.
Treatment protocolPatients were premedicated with methylprednisolone 125mg, dexchlorpheniramine 5mg, and paracetamol 1g intravenously the day of the infusion and the day after. A single cycle of two infusions of rituximab 500mg was administered with an interval of 14 days. Vitals and immediate adverse effects were monitored during infusion and the day after.
Follow-up and end pointsFrequency of follow-up visits and laboratory investigations were performed considering individual patients’ general condition. End points and outcome measures were defined according to the International Pemphigoid Committee8 as (a) “Disease control”: time at which new lesions or pruritus cease to form and established lesions begin to heal; (b) “End of consolidation phase”: time at which no new lesions or pruritus have developed for a minimum of 2 weeks and the majority (approximately 80%) of established lesions has healed; (c) “Complete remission during tapering”: absence of nontransient lesions while the patient is receiving more than minimal therapy; (d) “Complete remission”: absence of new or established lesions or pruritus for at least 2 months while the patient is off all BP therapy (CR OFF) or with minimal therapy (CR ON); (e) “Partial remission”: presence of transient new lesions that heal within 1 week for at least 2 months while the patient is off all BP therapy (PR OFF) or with minimal therapy (PR ON). “Minimal therapy” is defined as less than or equal to 0.1mg/kg/day of prednisone (or the equivalent) or 20g/week of clobetasol propionate and/or minimal adjuvant therapy (methotrexate 5mg/week; azathioprine 0.7mg/kg/day; mycophenolate mofetil 500mg/day; or dapsone 50mg/day).
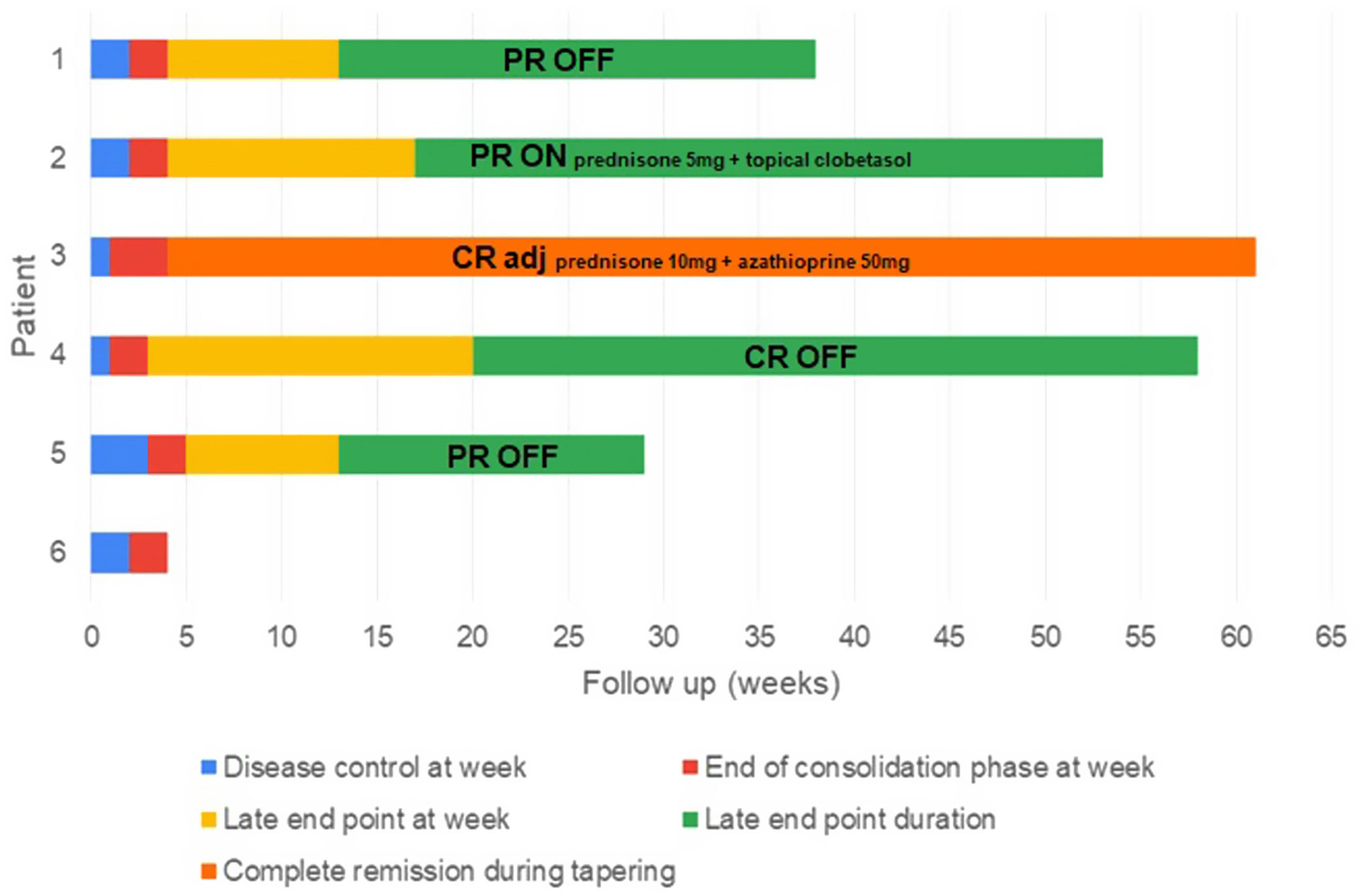
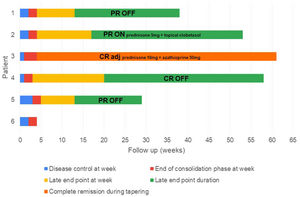
ResultsFive male and one female patients with a mean age of 78.6 years (range 65–89) were included. Their mean Charlson comorbidity index was 5.6 (range 3–9). The mean history of BP before rituximab treatment was 6.7 months (range 2–16), and it was associated with Alzheimer's disease in two patients and dipeptidyl peptidase-4 (DPP-4) inhibitors intake in one patient. Prior to rituximab, all patients had received conventional systemic therapy including prednisone (six patients), doxycycline (four patients) or azathioprine (three patients), in addition to clobetasol propionate cream 0.05% (six patients). The patients were followed-up for a mean of 52 weeks (range 6–77). Table 1 and Fig. 1 show patients’ characteristics and end points, respectively.
Demographic, clinical characteristics and outcomes of patients with bullous pemphigoid treated with low-dose rituximab.
| Patient | Sex/age (years) | History of the disease (months) | Associated conditions | Previous therapies | Adverse events of rituximab | Disease control at week | Late end point (at week) | Minimal therapy | Relapse at week | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/82 | 7 | Gliptin intake | AZA (50 mg), PRED (30 mg), CLOB | – | 2 | PR OFF (13) | – | – | 54 |
| 2 | M/65 | 16 | – | AZA (75 mg/day), DOXY (100 mg/day), PRED (20 mg/day), CLOB | Herpetic gingiva-stomatitis | 2 | PR ON (17) | PRED 5mg+CLOB | – | 69 |
| 3 | M/81 | 2.5 | – | PRED (30 mg/day), DOXY (100 mg/day) | – | 1 | – | – | 4 | 77 |
| 4 | M/89 | 6 | Alzheimer (GDS=4) | PRED (20mg/day), DOXY (100 mg/day), CLOB | – | 1 | CR OFF (20) | – | – | 74 |
| 5 | M/77 | 2 | Alzheimer (GDS=6) | PRED (30 mg/day), CLOB | – | 3 | PR OFF (19) | – | 32 | |
| 6 | F/77 | 6 | – | PRED (20 mg/day),CLOB | – | 2 | – | CLOB | – | 6 |
AZA, azathioprine; CLOB, clobetasol propionate cream 0.05%; CR OFF, complete remission off all therapy; DOXY, doxycycline; GDS: global deterioration scale; PRED: prednisone; PR OFF, partial remission off all therapy; PR ON, partial remission with minimal therapy.
Disease phases and end points after rituximab treatment. Each bar represents a patient and its length shows the duration of follow-up. After a single cycle of low-dose rituximab (at week 0), the time to disease control (blue), the time to end of consolidation (red), the time to late end point (yellow) and its duration (green) are shown. One patient had a relapse (black line), requiring retreatment with more than minimal adjuvant therapy (light blue).
CR adj: complete remission with more than minimal adjuvant therapy (also referred to “complete remission during tapering”); CR OFF: complete remission off all therapy; CR ON: complete remission with minimal therapy; PR OFF: partial remission off all therapy; PR ON: partial remission with minimal therapy.
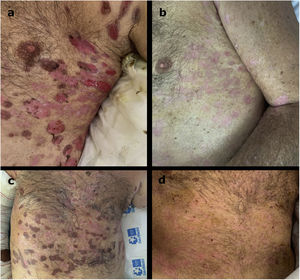
We observed a rapid and marked response after the two infusions of rituximab (probably influenced also by the premedication with methylprednisolone) (Fig. 2). The mean time to disease control was 1.9 weeks (range 1–3), and the mean time to end of consolidation phase was 4 weeks (range 3–5).
Late end pointsAfter a single cycle of low-dose rituximab, four patients (80%) achieved a late end point at a mean of 15.75 weeks (range 13–20), which was maintained at the time of writing this article, for a mean of 28.75 weeks (range 16–38). Three patients achieved partial remission: two with no need of any medication (PR OFF), and one with minimal therapy (PR ON) (prednisone 5mg plus clobetasol propionate cream 0.05%, and clobetasol propionate cream 0.05% alone, respectively). One patient achieved complete remission with no therapy (CR OFF). One patient has 6 weeks of follow-up.
The remaining patient achieved disease control at 1 week, but relapsed 4 weeks after the rituximab treatment. However, he remains in complete remission during tapering with more than minimal adjuvant therapy (CR adj) (prednisone 10mg/day and azathioprine 50mg/day).
Adverse eventsOne patient presented with oral erosions and vesicles 2 months after rituximab treatment. It was diagnosed with herpetic gingivostomatitis (confirmed by PCR) and resolved with oral valacyclovir. Another patient who had a claw hand secondary to a stroke developed a pressure ulcer requiring a finger amputation 1.5 months after rituximab treatment, yet a direct link with rituximab is unlikely. No other serious adverse events occurred during the follow-up.
DiscussionRituximab is a humanized chimeric monoclonal antibody directed against CD-20 antigen on B lymphocytes, with several approved and off-label indications. It has been recently approved by the US Food and Drug Administration (FDA) in 2018, and the European Medicines Agency (EMA) in 2019 as a treatment for PV. Since then, there are increasing articles reporting the treatment of BP with rituximab at standard doses (Table 2).2,5,6 Lymphoma protocol is more commonly used than RA protocol, although one study found no difference in the efficacy between them.6 At these standard doses, complete remission ranged between 35%2 and 85%5 of the patients, and partial remission between 15%5 and 40%.2 These studies reported serious adverse events, mainly infectious, and even cases of deaths. Overall, data suggest that rituximab at standard doses is effective in BP, and also useful as an steroid- and immunosuppressant-sparing therapy but may have serious adverse effects.2,5,6 In addition, a recently published study has shown that mortality in patients with BP treated with rituximab is lower than in those receiving standard treatment.9
Comparison of outcomes between treatment of BP with low-dose rituximab (current study) and standard doses.
| Current study, n=5 | Polansky et al., 2019; n=20 | Kremer et al., 2018; n=62 | Tovanabutra et al., 2019; n=21 | |
|---|---|---|---|---|
| Dose of RTX | 2 doses of 500mg (low-dose RTX) | 1: Lymphoma19: RA | 33: Lymphoma27: RA2: other | 14: Lymphoma7: RA |
| Complete remission, n (%) | 2 (40%)- CR OFF: 1 (20%)- CR adj: 1 (20%) | 7 (35%)- CR OFF: 2 (10%)- CR ON: 4 (20%)- CR adj: 1 (5%) | 52 (85%)a- CR OFF or ON: 40 (65%)- CR adj: 12 (20%) | 12 (57%)- CR OFF: 2 (9.5%)- CR ON: 10 (47.6%) |
| Partial remission, n (%) | 3 (60%)- PR OFF: 2 (40%)- PR ON: 1 (20%) | 8 (40%)- PR OFF: 1 (5%)- PR ON: 3 (15%)- PR adj: 4 (20%) | 9 (15%)Not specified | 4 (19%)- PR OFF: 1 (4.7%)- PR ON: 3 (14.3%) |
| Time to remission, mean (range); months | 3.67 (3–4.7) | 5.6 (1.9–13.9) | – | – |
| Relapse, n (%) | 1 (20%) | 2 (13.3%) | 15 (24.5%) | 16 (76.2%) |
| Time from RTX to relapse, mean (range); months | 1 | 11.8 (10.9–12.6) (from first dose of RTX) | 10.2 (1–24) (from last dose of RTX) | – |
| Follow-up period, mean (range); months | 11.2 (6.8–14.2) | 16.9 (4.4–41.6) | 33 (3–108) | 44.5 (3.7–101.5) |
| Adverse effects | 1 patient (20%),Mild | 23 events- 15 hospitalizations- 0 deaths | 13 patients (24%)- 2 deaths | 4 patients (19%)-1 death |
CR adj, complete remission with more than minimal adjuvant therapy; CR OF, complete remission off all therapy; CR ON, complete remission with minimal therapy; PR adj, partial remission with more than minimal therapy; PR OFF, partial remission off all therapy; PR ON, partial remission with minimal therapy. Lymphoma protocol (375mg/m2 weekly for 4 weeks); RA: rheumatoid arthritis protocol (two infusions of 1000mg repeated in 2 weeks); RTX: rituximab.
This study classified as CR OFF patients who were off therapy or treated with prednisone with a maximal dose of 10mg. Since the latter group is better defined as CR ON, this group is expressed in the table as CR OFF or ON. Also this study classified CR ON as patients with more than prednisone 10mg and/or other systemic therapies. This group is expressed in the table as CR adj.
Low-dose rituximab has been reported to be effective in a myriad of autoimmune diseases, such as myasthenia gravis, rheumatoid arthritis or immune thrombocytopenia.10 In Dermatology, low-dose rituximab has been mainly used in PV, with satisfactory data in effectiveness and safety.3,4 A meta-analysis by Wang et al. found no difference between standard doses and low-dose in time to disease control, complete remission rate and relapse rate in patients with PV.11
In our study, we present six patients with BP treated with low-dose rituximab (two infusions of 500mg), which is 39% of the dose used in hematology and 50% of the dose used in rheumatology, with an acceptable effectiveness and safety profile. The administration of high doses of methylprednisolone has probably produced this rapid beneficial effect as observed in other diseases,12 but good long-term control of the disease is derived from the use of rituximab. Only 1 patient of our series experienced a mild adverse event, compared to several serious adverse events with standard doses.2,5,6 CR (including off therapy, minimal therapy and more than minimal therapy) was achieved in 40% of the patients (compared to 352–85%5 with standard doses), and PR in 60% of the patients (compared to 155–40%2 with standard doses) (Table 2). Only 1 patient relapsed and had to continue with immunosuppressive therapy, supporting that low-dose rituximab is also useful to avoid steroids and immunosuppressants, decreasing the morbidity and adverse events in advanced age BP patients.
We highlight that these are dependent patients, with high comorbidity and polymedication, leading to a low adherence to first-line treatment (high-potency topical corticosteroids). The aim of this treatment is to improve the quality of life of the patients, reducing their needed care without increasing side effects.
Our study had several limitations, including its retrospective nature, small sample size and a short follow-up period that does not allow to see the effects in the very long term. However, to our knowledge this is the first series of BP treated with low-dose rituximab and it will be useful for future prospective studies.
In conclusion, low-dose rituximab for the treatment of BP achieved acceptable remission rates and steroid-sparing activity, with a better safety profile and a lower cost, compared to standard doses. Although future studies are needed, this pilot study suggests that low-dose rituximab could be a therapeutic option for BP.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.