Dermatologists’ interest in the Janus-associated kinase/signal transducers and activators of transcription (JAK/STAT) pathway has been growing as evidence builds to support its key role in the pathogenesis of inflammatory skin diseases. Because certain proinflammatory cytokines use the JAK/STAT pathway for signal transduction, it has become a promising therapeutic target in diseases where selective modulation of the immune system can be useful.
We aim to review current knowledge of the JAK/STAT signaling pathway and its role in immune-mediated skin diseases. In the first part of the review we cover the efficacy and safety of oral and topical JAK inhibitors in the treatment of vitiligo and alopecia areata.
La vía de señalización de citocinas Janus cinasa/transductor de señal y activador de transcripción (JAK/STAT) es un área de interés emergente en dermatología, con evidencia creciente del papel clave en la patogénesis de las enfermedades inflamatorias cutáneas. Debido a que algunas citocinas proinflamatorias usan la vía JAK/STAT para la transducción de señales, ésta se convierte en una diana terapéutica prometedora para el tratamiento de dichas enfermedades, al modular de forma selectiva el sistema inmune.
El objetivo de esta revisión es conocer la vía de señalización JAK/STAT y su papel en distintas enfermedades dermatológicas inmunomediadas. En esta primera parte, se revisará la eficacia y seguridad de los inhibidores de JAK –en formulación oral o tópica– para el tratamiento del vitíligo y la alopecia areata.
The intracellular Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is activated by binding of an extracellular ligand to various transmembrane receptors, leading to phosphorylation of intracellular molecules and initiation of an intracellular signaling cascade and, eventually, regulation of the transcription of numerous genes. The Janus kinase (JAK) family comprises tyrosine kinases that act within the cell as signal transducers and include the molecules JAK1, JAK2, JAK3, and TYK2.
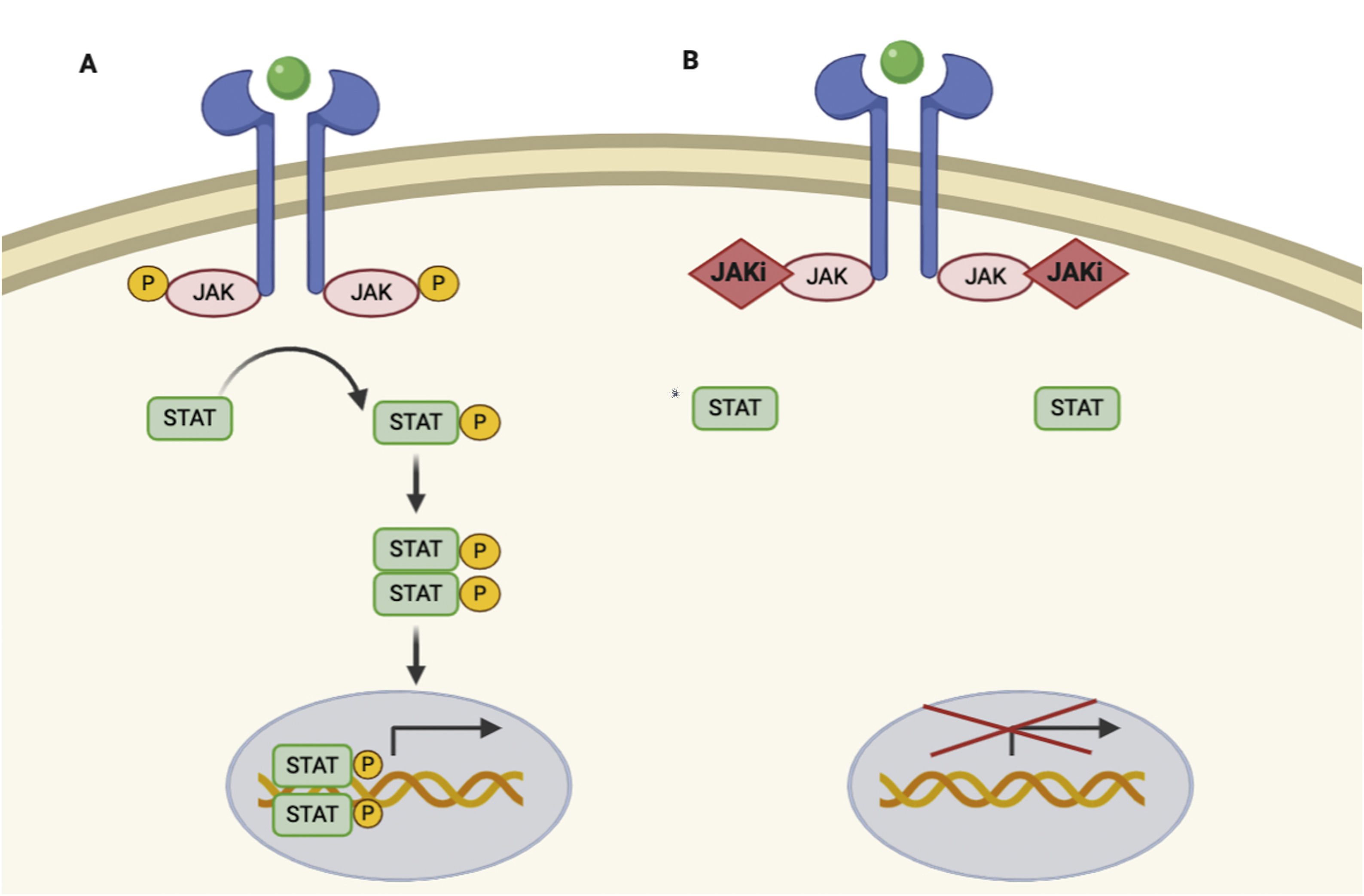
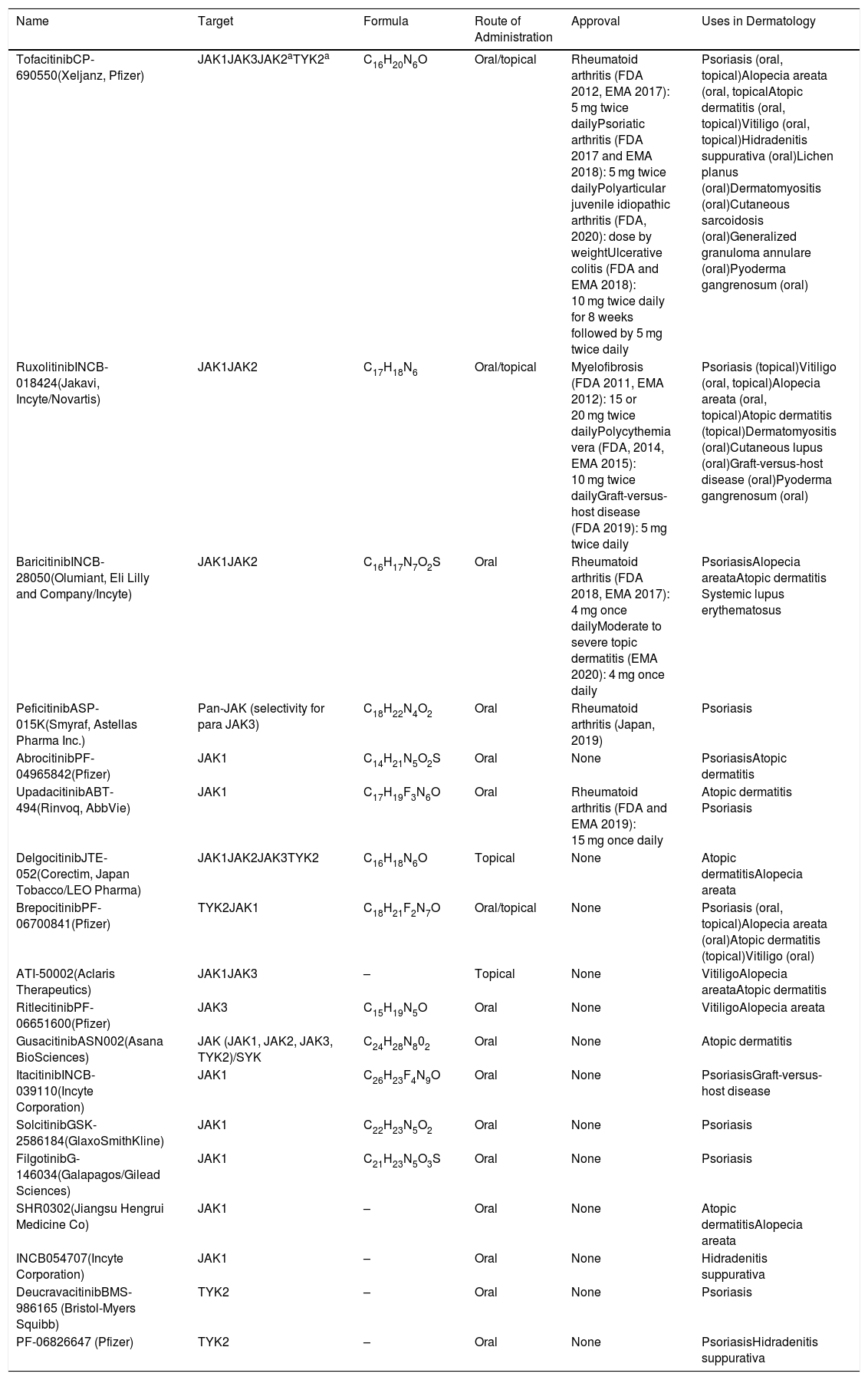
JAKs act by forming dimers in the intracytoplasmic portion of the cytokine receptors. These JAK dimers can combine with multiple receptors and become activated by various cytokines and in turn activate STAT proteins (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6),1 thus enabling them to participate in specific biological functions (Table 1). On activation, STAT proteins combine to form dimers and can translocate to the cell nucleus, where they can act as transcription factors—upregulating the genes responsible for production of proinflammatory cytokines and growth factors—or regulate the behavior of other intracellular proteins (Fig. 1A).2
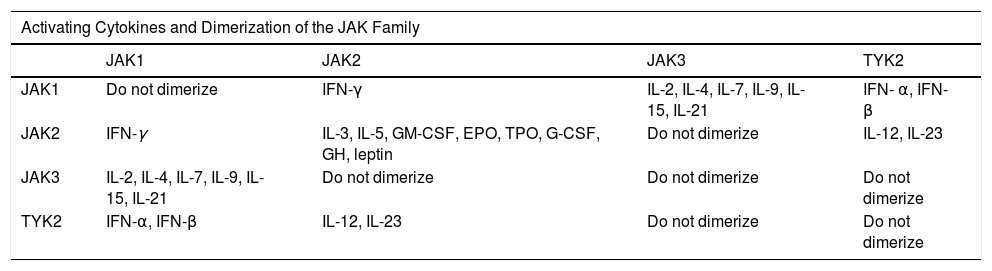
The Janus Kinase (JAK) Family.
| Activating Cytokines and Dimerization of the JAK Family | ||||
|---|---|---|---|---|
| JAK1 | JAK2 | JAK3 | TYK2 | |
| JAK1 | Do not dimerize | IFN-γ | IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 | IFN- α, IFN- β |
| JAK2 | IFN-γ | IL-3, IL-5, GM-CSF, EPO, TPO, G-CSF, GH, leptin | Do not dimerize | IL-12, IL-23 |
| JAK3 | IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 | Do not dimerize | Do not dimerize | Do not dimerize |
| TYK2 | IFN-α, IFN-β | IL-12, IL-23 | Do not dimerize | Do not dimerize |
Abbreviations: EPO, erythropoietin; G-CSF, granulocyte colony-stimulating factor; GH, growth hormone; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-α, interferon-alfa; IFN-β, interferon-beta; IFN-γ, interferon-gamma; TPO, thrombopoietin.
All members of the JAK family are ubiquitous in mammals, except JAK3, which is expressed mainly in lymphoid and hematopoietic tissue. Each member of the JAK family dimerizes with another member of the same family and is activated after binding of specific cytokines to transmembrane receptors. For example, IFN-γ depends on JAK1/JAK2 signaling and participates in T-cell differentiation, lymphocyte effector function, and macrophage activation. JAK2/JAK2 participate in myeloid and lymphoid differentiation, proliferation and survival of T cells, lymphocyte effector function, hematopoiesis, growth, and anabolic metabolism. JAK2/TYK2 intervene in T-cell differentiation and in lymphocyte effector function. JAK1/JAK3 participate in the proliferation and survival of T cells and memory T cells and in the function of regulatory T cells.
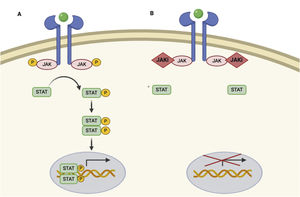
JAK/STAT pathway and mechanism of action of JAK inhibitors.
A, JAK/STAT pathway. The cytokine binds to the receptor, thus activating JAK proteins via phosphorylation. The activated JAK protein phosphorylates the STAT protein, in turn activating it. Activated STAT is translocated to the nucleus. Activated STAT protein acts as a transcription factor and binds to DNA, thus regulating the transcription of a wide variety of genes and affecting cell growth and apoptosis. B, Mechanism of action of JAK inhibitors. The inhibitors bind to the binding site of adenosine triphosphate of the JAK dimer, thus preventing autophosphorylation and activation. Similarly, without activation of JAK, the protein STAT cannot be activated or translocated to the nucleus, resulting in reduced transcription of proinflammatory genes. Figure generated with the assistance of Biorender.com. JAK indicates Janus kinase; JAKi, JAK inhibitor; STAT, signal transducer and activator of transcription.
Given that the immune response is coordinated and regulated by soluble mediators, most of which are proinflammatory cytokines, the JAK/STAT pathway is a therapeutic target in various immune-mediated inflammatory diseases. JAK inhibitors are small molecules that inhibit the kinase activity of JAK and effectively diminish intracellular transduction of the JAK/STAT pathway (Fig. 1B).
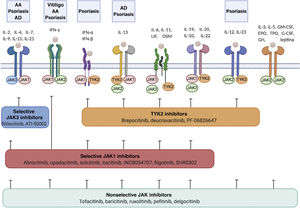
In recent years, multiple JAK inhibitors have demonstrated their efficacy in diseases such as rheumatoid arthritis, myelofibrosis, and polycythemia vera. First-generation JAK inhibitors, such as ruxolitinib, baricitinib, delgocitinib, and tofacitinib, are poorly selective and inhibit various JAKs, whereas the second-generation inhibitors, such as ritlecitinib, deucravacitinib, upadacitinib, and abrocitinib, are more selective and predominantly block (sometimes exclusively) a single member of the JAK family, thus inhibiting a narrower range of cytokines (Table 2, Fig. 2).
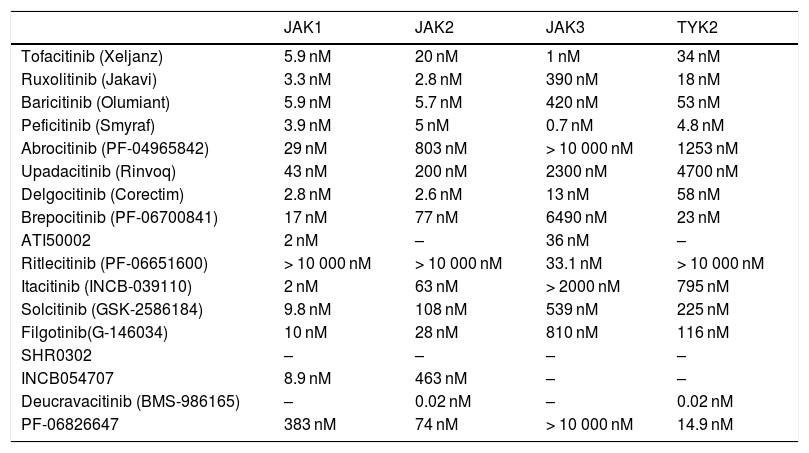
Selectivity of the JAK Inhibitors Used in Dermatology (IC50).a
| JAK1 | JAK2 | JAK3 | TYK2 | |
|---|---|---|---|---|
| Tofacitinib (Xeljanz) | 5.9 nM | 20 nM | 1 nM | 34 nM |
| Ruxolitinib (Jakavi) | 3.3 nM | 2.8 nM | 390 nM | 18 nM |
| Baricitinib (Olumiant) | 5.9 nM | 5.7 nM | 420 nM | 53 nM |
| Peficitinib (Smyraf) | 3.9 nM | 5 nM | 0.7 nM | 4.8 nM |
| Abrocitinib (PF-04965842) | 29 nM | 803 nM | > 10 000 nM | 1253 nM |
| Upadacitinib (Rinvoq) | 43 nM | 200 nM | 2300 nM | 4700 nM |
| Delgocitinib (Corectim) | 2.8 nM | 2.6 nM | 13 nM | 58 nM |
| Brepocitinib (PF-06700841) | 17 nM | 77 nM | 6490 nM | 23 nM |
| ATI50002 | 2 nM | – | 36 nM | – |
| Ritlecitinib (PF-06651600) | > 10 000 nM | > 10 000 nM | 33.1 nM | > 10 000 nM |
| Itacitinib (INCB-039110) | 2 nM | 63 nM | > 2000 nM | 795 nM |
| Solcitinib (GSK-2586184) | 9.8 nM | 108 nM | 539 nM | 225 nM |
| Filgotinib(G-146034) | 10 nM | 28 nM | 810 nM | 116 nM |
| SHR0302 | – | – | – | – |
| INCB054707 | 8.9 nM | 463 nM | – | – |
| Deucravacitinib (BMS-986165) | – | 0.02 nM | – | 0.02 nM |
| PF-06826647 | 383 nM | 74 nM | > 10 000 nM | 14.9 nM |
Abbreviations: IC50, concentration of drug that leads to 50% inhibition; JAK, Janus kinase.
The table shows the IC50 values for inhibition of JAK1, JAK2, JAK3, and TYK2 by the various JAK inhibitors used in dermatology.2,96,97 Given that gusacitinib (ASN002) inhibits SYK, JAK1, JAK2, JAK3, and TYK2, it is not shown in the table. Its IC50 values are 5, 46, 4, 11, and 8 nM, respectively.98,99
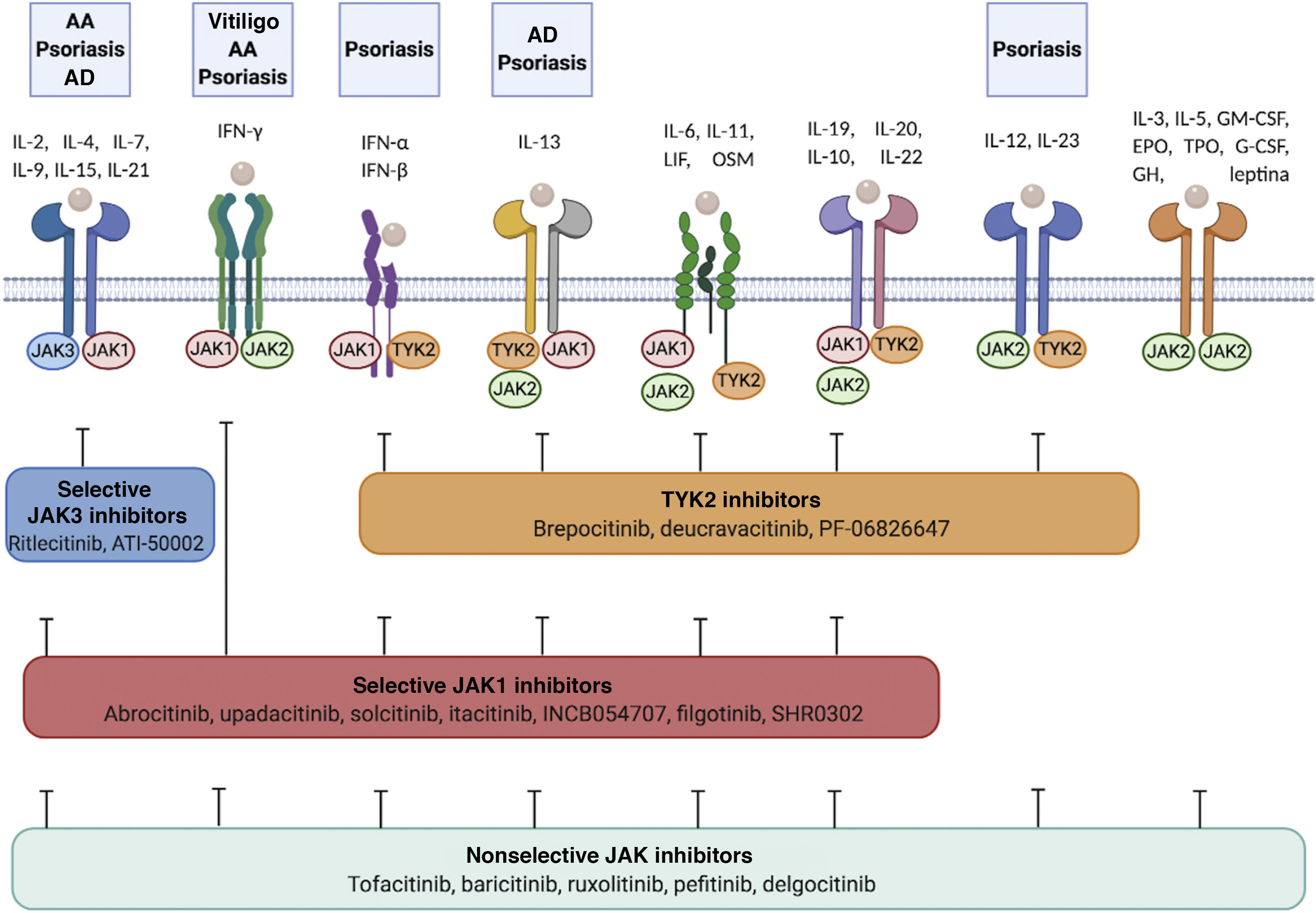
Cytokine receptors and selectivity of JAK inhibitors in dermatology.
Various cytokine receptors bind to JAK proteins, which transduce the extracellular ligand signals within the cell. JAK inhibitors have different capacities for blocking cytokine receptor signaling: nonselective inhibitors inhibit many cytokines simultaneously, whereas more selective JAK inhibitors inhibit a specific biological function but enable signaling of other cytokines via JAK-dependent pathways. Figure generated with the assistance of Biorender.com.
Abbreviations: AA indicates alopecia areata; AD, atopic dermatitis; EPO, erythropoietin; G-CSF, granulocyte colony-stimulating factor; GH, growth hormone; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-α, interferon alfa; IFN-β, interferon beta; IFN-γ, interferon gamma; IL, interleukin; JAK, Janus kinase; LIF, leukemia inhibitory factor; OSM, oncostatin M; TYK, tyrosine kinase; TPO, thrombopoietin.
JAK inhibitors can broaden options for therapy in various cutaneous inflammatory diseases (Table 3, Fig. 2). By reducing the effect of all the cytokines that activate the corresponding JAK/STAT pathway, JAK inhibitors could prove more effective than classic biologics, which only target a single cytokine.3 Another advantage of JAK inhibitors is their small size, which facilitates penetration of the epidermal barrier, thus enabling them to be used in topical formulations.4
Main JAK Inhibitors and Use in Dermatology.
| Name | Target | Formula | Route of Administration | Approval | Uses in Dermatology |
|---|---|---|---|---|---|
| TofacitinibCP-690550(Xeljanz, Pfizer) | JAK1JAK3JAK2aTYK2a | C16H20N6O | Oral/topical | Rheumatoid arthritis (FDA 2012, EMA 2017): 5 mg twice dailyPsoriatic arthritis (FDA 2017 and EMA 2018): 5 mg twice dailyPolyarticular juvenile idiopathic arthritis (FDA, 2020): dose by weightUlcerative colitis (FDA and EMA 2018): 10 mg twice daily for 8 weeks followed by 5 mg twice daily | Psoriasis (oral, topical)Alopecia areata (oral, topicalAtopic dermatitis (oral, topical)Vitiligo (oral, topical)Hidradenitis suppurativa (oral)Lichen planus (oral)Dermatomyositis (oral)Cutaneous sarcoidosis (oral)Generalized granuloma annulare (oral)Pyoderma gangrenosum (oral) |
| RuxolitinibINCB-018424(Jakavi, Incyte/Novartis) | JAK1JAK2 | C17H18N6 | Oral/topical | Myelofibrosis (FDA 2011, EMA 2012): 15 or 20 mg twice dailyPolycythemia vera (FDA, 2014, EMA 2015): 10 mg twice dailyGraft-versus-host disease (FDA 2019): 5 mg twice daily | Psoriasis (topical)Vitiligo (oral, topical)Alopecia areata (oral, topical)Atopic dermatitis (topical)Dermatomyositis (oral)Cutaneous lupus (oral)Graft-versus-host disease (oral)Pyoderma gangrenosum (oral) |
| BaricitinibINCB-28050(Olumiant, Eli Lilly and Company/Incyte) | JAK1JAK2 | C16H17N7O2S | Oral | Rheumatoid arthritis (FDA 2018, EMA 2017): 4 mg once dailyModerate to severe topic dermatitis (EMA 2020): 4 mg once daily | PsoriasisAlopecia areataAtopic dermatitis Systemic lupus erythematosus |
| PeficitinibASP-015K(Smyraf, Astellas Pharma Inc.) | Pan-JAK (selectivity for para JAK3) | C18H22N4O2 | Oral | Rheumatoid arthritis (Japan, 2019) | Psoriasis |
| AbrocitinibPF-04965842(Pfizer) | JAK1 | C14H21N5O2S | Oral | None | PsoriasisAtopic dermatitis |
| UpadacitinibABT-494(Rinvoq, AbbVie) | JAK1 | C17H19F3N6O | Oral | Rheumatoid arthritis (FDA and EMA 2019): 15 mg once daily | Atopic dermatitis Psoriasis |
| DelgocitinibJTE-052(Corectim, Japan Tobacco/LEO Pharma) | JAK1JAK2JAK3TYK2 | C16H18N6O | Topical | None | Atopic dermatitisAlopecia areata |
| BrepocitinibPF-06700841(Pfizer) | TYK2JAK1 | C18H21F2N7O | Oral/topical | None | Psoriasis (oral, topical)Alopecia areata (oral)Atopic dermatitis (topical)Vitiligo (oral) |
| ATI-50002(Aclaris Therapeutics) | JAK1JAK3 | – | Topical | None | VitiligoAlopecia areataAtopic dermatitis |
| RitlecitinibPF-06651600(Pfizer) | JAK3 | C15H19N5O | Oral | None | VitiligoAlopecia areata |
| GusacitinibASN002(Asana BioSciences) | JAK (JAK1, JAK2, JAK3, TYK2)/SYK | C24H28N802 | Oral | None | Atopic dermatitis |
| ItacitinibINCB-039110(Incyte Corporation) | JAK1 | C26H23F4N9O | Oral | None | PsoriasisGraft-versus-host disease |
| SolcitinibGSK-2586184(GlaxoSmithKline) | JAK1 | C22H23N5O2 | Oral | None | Psoriasis |
| FilgotinibG-146034(Galapagos/Gilead Sciences) | JAK1 | C21H23N5O3S | Oral | None | Psoriasis |
| SHR0302(Jiangsu Hengrui Medicine Co) | JAK1 | – | Oral | None | Atopic dermatitisAlopecia areata |
| INCB054707(Incyte Corporation) | JAK1 | – | Oral | None | Hidradenitis suppurativa |
| DeucravacitinibBMS-986165 (Bristol-Myers Squibb) | TYK2 | – | Oral | None | Psoriasis |
| PF-06826647 (Pfizer) | TYK2 | – | Oral | None | PsoriasisHidradenitis suppurativa |
Abbreviations: EMA, European Medicines Agency; FDA, United States Food and Drug Administration; SYK, spleen tyrosine-kinase.
JAK inhibitors have an acceptable risk-benefit ratio, although in most cases, the results come from studies carried out in inflammatory diseases such as rheumatoid arthritis. Reported adverse effects are mainly mild to moderate,5–10 with the most frequent being upper respiratory tract, urinary tract, and gastrointestinal tract infections. An increased risk of reactivation of herpes zoster virus has been observed in patients treated with baricitinib,11 doubling that of the biologic tumor necrosis factor inhibitors.12,13 Tofacitinib has been associated with the development of herpes zoster infection in 1%-3% of cases.14–16 In addition, there have been reports of reactivation of tuberculosis with tofacitinib and baricitinib.5,12,14–16
The results of a trial on rheumatoid arthritis comparing tofacitinib 5 mg or 10 mg twice daily with etanercept, a tumor necrosis factor inhibitor, revealed a greater risk of thrombosis in the group taking 10 mg twice daily than in the placebo group; however, further studies are needed to confirm this finding.17 Various meta-analyses and systematic reviews did not find an increased incidence of neoplasm with tofacitinib.18–20 Other studies report the incidence of neoplasm to be similar to that associated with biologics.19,21 However, aggressive B-cell lymphoma has been observed in patients with myeloproliferative tumors treated with ruxolitinib.22
Inhibition of JAK2 interferes with erythropoiesis, myelopoiesis, and platelet activation; therefore, it carries a risk of anemia, neutropenia, and thrombocytopenia.7,8,23–26 Given its high potency as a JAK2 inhibitor, ruxolitinib leads more frequently to myelosuppression than other JAK inhibitors. Inhibition of JAK1 has been associated with an increase in levels of total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides,8,24,27–30 although these return to normal values after 1-3 months. We can also observe a transient increase in levels of liver enzymes, creatine phosphokinase, and creatinine, which is reversible when the drug is discontinued.24,27,28,31 The loss of TYK2 activity can increase the risk of severe cutaneous infection by herpesvirus, staphylococci, and mycobacteria.32
The safety profile of topical JAK inhibitors is better than that of the oral drugs owing to their minimal systemic absorption.9,33–35 These agents have been used as cream and ointment and in a liposomal base and can cause cutaneous irritation and folliculitis depending on the vehicle used. A study on ruxolitinib cream reported the appearance of erythema (72%), hyperpigmentation, and transient acne.33
JAK Inhibitors in DermatologyDysregulation of the JAK/STAT pathway has been observed in various inflammatory dermatologic diseases, with differences in expression of JAK in biopsies from both healthy and affected skin.36
JAK molecules are overexpressed in the epidermis, dermis, or both in psoriasis, cutaneous lupus erythematosus, pyoderma gangrenosum, atopic dermatitis, and alopecia areata.36 JAK3 expression is markedly increased in the epidermis in these diseases except for lupus, with potential implications for therapy in terms of the use of topical JAK3 inhibitors. For example, topical tofacitinib has been used for the treatment of psoriasis, alopecia areata,37–39 and atopic dermatitis,9 whereas topical delgocitinib has been trialed for treatment of atopic dermatitis.40,41 In the case of cutaneous lupus erythematosus, on the other hand, an increase in JAK1 activity has been shown in the dermis, and selective JAK1 inhibitors could be useful for treatment of this condition.42 JAK1, JAK2, and JAK3 are overexpressed in the dermis in pyoderma gangrenosum and atopic dermatitis, thus indicating a possible therapeutic role for pan-JAK inhibitors, such as tofacitinib.
Below, we describe in detail the role of the JAK-STAT pathway in vitiligo and alopecia areata and present the results obtained with JAK inhibitors in various studies. Similarly, part 2 of this review will examine pathogenesis in depth and discuss the role of JAK inhibitors in psoriasis, atopic dermatitis, and other skin diseases.
VitiligoElevated levels of interferon γ (IFN-γ) and its associated cytokines CXCL9 and CXCL10 have been reported in human skin affected by vitiligo. Via JAK1/2,43 IFN-γ activates transcription of CXCL9 and CXCL10, which are necessary for the recruitment of cytotoxic T lymphocytes, the end effector cells in the destruction of melanocytes (Fig. 2). Inhibition of JAK proteins could be an effective therapeutic strategy for the treatment of vitiligo, thus reducing production of CXCL9 and CXCL10.44,45
A recent meta-analysis of 45 patients with vitiligo treated with JAK inhibitors revealed repigmentation > 50% in 57.8% of patients; this reached 70% in the case of facial vitiligo and 88.9% when concomitant phototherapy was used.46
However, when treatment with JAK inhibitors is interrupted, patients experience relapses, which may be determined by the presence of self-reactive memory cells in the skin (tissue-resident memory cells). IL-15, whose expression is increased in the epidermis in vitiligo,47 plays a key role in the maintenance of tissue-resident memory CD8+ cells, suggesting that it could be an effective targeted treatment in patients with vitiligo.48
Oral TofacitinibTofacitinib (Xeljanz, Pfizer) is a JAK1/3 inhibitor that has proven effective in case reports or series of cases of vitiligo. The dose of 5 mg/d led to almost complete repigmentation of the face and hands in a patient with progressive vitiligo at 5 months and with no adverse effects; however, the improvement was not sustained when treatment was suspended.49 In a patient with multifocal nonsegmental vitiligo who started tofacitinib 5 mg twice daily for 6 months for treatment of concomitant atopic dermatitis, vitiligo improved slightly (reduction in the Vitiligo Area Scoring Index [VASI] from 4.68 at baseline to 3.95 at 5 months). The patient experienced 2 episodes of upper respiratory tract infection and 1 of diarrhea that did not require treatment to be interrupted.50
Repigmentation of patients with vitiligo treated with JAK inhibitors could require photostimulation to induce migration of melanocytes to the epidermis. In a retrospective series of 10 patients receiving tofacitinib 5-10 mg once or twice daily, repigmentation only occurred when the drug was administered concomitantly with exposure to sunlight or narrowband UV-B (NB-UVB).51 A multicenter retrospective study found that repigmentation rates were higher in patients treated with NB-UVB and tofacitinib 10 mg/d than in those treated with phototherapy alone (92 vs 77%).52
The autoimmune response has been shown to be suppressed both in areas exposed to light (responders) and in those not exposed (nonresponders), thus suggesting a model where JAK inhibitors suppress the inflammatory response induced by the subtype 1 helper T-cell inflammatory response and phototherapy activates regeneration of melanocytes.51 This combination requires a lower dose of light exposure.
Topical TofacitinibA pilot study analyzed 16 patients with nonsegmental vitiligo treated with tofacitinib 2% cream, of whom 13 experienced repigmentation. The mean follow-up was 153 days (range, 63-367 days). The response was more marked for facial lesions and in Fitzpatrick skin types IV-VI, whereas the mean frequency of repigmentation in nonfacial lesions was 16%.53 One of the patients had acne as an adverse effect.
Topical tofacitinib also seems to lead to greater repigmentation with exposure to light. The facial VASI score improved by 70% in a series of 11 patients with facial vitiligo treated with topical tofacitinib 2% cream twice daily, together with NB-UVB 3 times per week for 2–4 months. While these results point to a synergetic relationship, studies on extrafacial vitiligo are necessary.54
Oral RuxolitinibRuxolitinib (Jakavi, Incyte/Novartis), a JAK1/2 inhibitor, was used to treat a male with alopecia areata and vitiligo at a dose of 20 mg twice-daily for 20 weeks. Facial repigmentation started at 12 weeks, reaching 51% at 20 weeks (vs 0.8% at baseline).55
Topical RuxolitinibJoshipura et al.56 reported higher repigmentation rates in patients treated with ruxolitinib cream 1.5% twice daily on light-exposed areas compared with nonexposed areas. A statistically significant improvement in the overall VASI score was observed in patients with vitiligo who received ruxolitinib cream 1.5% twice daily and optional NB-UVB, with a particularly marked response in the case of facial vitiligo. The response was maintained at 6 months after discontinuation of treatment.57
The efficacy and safety profile of topical ruxolitinib 1.5% in cream twice daily for treatment of vitiligo is being evaluated in ongoing clinical trials (NCT0405242558 and NCT0405757359), as is the response to different doses (NCT0309930460).
Other JAK InhibitorsA topical JAK1/3 inhibitor (ATI-50002) for treatment of nonsegmental vitiligo is currently being evaluated in a clinical trial (NCT0346885561). Ritlecitinib (JAK3 inhibitor) and brepocitinib (JAK1 and TYK2 inhibitor) combined with phototherapy are also being evaluated in patients with active nonsegmental vitiligo (NCT0371582962).
Alopecia AreataThe pathogenesis of alopecia areata is complex. Vδ1+ T cells may be involved in the early stages of the disease, since it has been observed that the Vδ1+ T-cell count is considerably higher in both affected hair follicles and those that have not yet been damaged.63 There is also evidence that cytotoxic CD8+NKG2D+ T cells could play a key role in the pathogenesis of alopecia areata in animal models; in this case, the potential objective could be follicles that lose their immune privilege during the anagen phase.64 CD8+NKG2D+ T cells produce IFN-γ, which binds to its receptor on the surface of the hair follicles and activates the JAK1/2-STAT1 pathway. This in turn leads to production of IL-15, which, on binding to its receptor on the surface of the T cells, activates JAK1/3-STAT5, thus generating production of IFN-γ and perpetuating the inflammatory response (Fig. 2).65 Analysis of skin biopsies from patients with alopecia areata has revealed overexpression of JAK3 and, albeit to a lesser extent, JAK1 and JAK2.36 Concomitantly, we can observe overregulation of various cytokines—including IL-2, IL-7, and IL-21—which signal via JAK1/JAK3 and promote activation and survival of CD8+NKG2D+ T cells.64
Given the role of JAK in the pathogenesis of alopecia areata, JAK inhibitors could prove effective for treatment. They could also exert a direct effect on follicles during the telogen phase, thus promoting re-entry into the anagen phase.64
A recent meta-analysis66 covering 289 patients with alopecia areata reported a response rate of 72.4% with JAK inhibitors, with a mean (SD) time to complete growth of 6.7 (2.2) months. The oral route was more effective than the topical route, with a probability of response that was 4-fold higher. Patients who have had the disease for longer have a diminished response to JAK inhibitors,67 and alopecia areata recurs 3 months after stopping treatment. Some areas, such as the eyelashes, eyebrows, beard, and body hair grow more quickly.68–70
Oral TofacitinibThe efficacy of oral tofacitinib in alopecia areata was first reported in a patient with alopecia universalis who started treatment with tofacitinib 15 mg/d for concomitant psoriasis and whose hair had completely regrown after 8 months of treatment.71 Since then, there have been reports of response in other patients with alopecia areata,72 some of whom had concomitant psoriasis,73 atopic dermatitis, or vitiligo,50,74 with simultaneous improvement of both diseases.
An open-label study of 66 patients with alopecia areata and hair loss of more than 50%, alopecia totalis, or alopecia universalis subsequently found that tofacitinib 5 mg/d twice daily for 3 months led to a change in the Severity of Alopecia Tool (SALT) score of between 5% and 50% in 32% of patients and of > 50% in 32%. However, the mean duration of the response until recurrence after discontinuation of treatment was 8.5 weeks. Infections were reported in 25.8%, mainly upper respiratory tract infection, although 2 patients had urinary tract infection, 1 developed bronchitis, and 1 had herpes zoster infection. None of the patients had to be admitted to hospital.75
Cheng et al.76 reported the cases of 11 patients with alopecia totalis/alopecia universalis treated with tofacitinib at between 5 mg/d and 11 mg/d (extended release) twice daily for a mean period of 14.4 months (range, 4.5-27 months) whose SALT score improved by a mean of 61.2% over baseline. The disease resolved completely in half of the patients, of whom some benefited from combination with intralesional triamcinolone applied to refractory areas. The adverse effects were dyslipidemia, gastrointestinal symptoms, and mild acne.
Liu et al.67 performed a retrospective study of 90 patients with alopecia areata affecting ≥ 40% of the scalp, alopecia totalis, or alopecia universalis treated with tofacitinib at ≥ 5 mg twice daily in monotherapy or combined with prednisone for a minimum of 4 months. In the 65 patients considered potential responders because they had alopecia totalis/alopecia universalis for under 10 years, the SALT score fell by >90% in 20%, by 51%-90% in 38.4%, and by 6%-50% in 18.5%. The most frequent adverse event was upper respiratory tract infection, which affected 28.9% of patients, although the authors also recorded a patient with bronchitis and 3 patients with urinary tract infection.
In a recent study of 12 patients with moderate to severe alopecia areata, alopecia totalis, or alopecia universalis, most cases required the dose of tofacitinib to be increased from 5 mg/12 h to 10 mg/12 h in order to achieve a response, with no increase in adverse effects. A response was recorded in 91.7% of patients, with a mean reduction in the SALT score of 56.8% at 32 weeks. However, when treatment was discontinued in the responders, values worsened compared with baseline in 50% after 6 months of observation.77 There have been multiple examples of patients with an initial response that was not maintained after interruption of treatment,68,78,79 with a return to levels of alopecia that were similar to or even worse than the baseline values.80 It is not clear whether this represents an increase in disease activity or whether discontinuation of treatment triggers a telogen effluvium, since JAK inhibitors promote re-entry into anagen.81
As for children and adolescents, there have been reports of cases of alopecia totalis and alopecia universalis treated satisfactorily with tofacitinib 5 mg/12 h, with no notable adverse effects.82,83
An ongoing cohort study is evaluating the safety and efficacy of tofacitinib 5 mg twice daily, together with the economic impact of the change in the quality of life of patients with extensive alopecia areata (NCT0380097984).
Topical TofacitinibTopical JAK inhibitors could prove useful, especially for the treatment of localized alopecia areata. Almost complete regrowth of the hair has been observed with topical tofacitinib 2% every 12 hours for 7 months76 and of the eyelashes after a mean 7 months of treatment (range, 3-11 months),39 with no adverse effects. However, a pilot study of 10 patients treated with tofacitinib ointment 2% twice daily revealed poor results, with only 3 responders and a mean decrease in the SALT score of 34.6% at 24 weeks.37 The adverse effects observed were local irritation (40%), folliculitis (10%), and increased cholesterol (40%).
Tofacitinib ointment 1%-2% was also reported to be efficacious in a group of adolescents after 3-9 months of treatment, with no remarkable adverse effects.34 Furthermore, use of tofacitinib 2% in a liposomal base yielded a variable response in 11 pediatric patients, although the response was cosmetically acceptable in most cases. Therefore, this option could be used as adjunctive second-line treatment in these patients.38
Oral RuxolitinibA woman treated with oral ruxolitinib 15 mg twice daily for thrombocytopenia experienced almost complete regrowth of alopecia universalis at 10 months, and the response was maintained with treatment at 50 months.85 In another case, involving a patient with alopecia areata and vitiligo, both conditions improved with ruxolitinib 20 mg twice daily, with initiation of growth at 4 weeks and a significant improvement at 12 weeks.55
In a study of 12 patients treated with ruxolitinib 20 mg/12 h for 3-6 months, 75% achieved a response, which took the form of an improvement in the mean baseline SALT score from 65.8% to 7.3% after 6 months of treatment. Most responders had regrowth of 95%, which was noticeable at 4 weeks of treatment.86 However, the response was lost 3 weeks after suspending treatment. The most common adverse effects were upper respiratory tract infection, although the authors also reported a case of mild pneumonia. The same dose was used to treat a preadolescent male with alopecia totalis.87 At 4 months, his eyebrows and scalp hair had almost regrown; therefore, the dose was reduced to 10 mg every other day, with no loss of efficacy. Ruxolitinib 30 mg/d has also been associated with complete regrowth at 8 months in a patient with alopecia totalis. Gradual reduction of the dose was not associated with a loss of response, although the patient developed transient anemia at initiation of treatment.88
While ruxolitinib 20 mg twice daily and tofacitinib 5 mg twice daily were not associated with significant differences in terms of regrowth after 6 months of treatment or in terms of the recurrence rate, initial regrowth seems to be earlier with ruxolitinib.89
Topical RuxolitinibVarying responses have been reported for topical ruxolitinib 0.6%-2%.35,90 In a prospective phase 1 trial, partial regrowth was observed at 28 weeks in 5 patients with alopecia universalis in the areas treated with topical ruxolitinib 1%.91
In the case of an adolescent with universal alopecia areata who applied 0.6% cream twice daily, complete growth of the eyebrows was observed at 3 months, albeit with only 10% regrowth on the scalp. No adverse events were reported. In children, the liposomal formulation is more effective, although the nonliposomal formulation is more acceptable in cosmetic terms.34
Oral BaricitinibBaricitinib (Olumiant, Eli Lilly and Company) is a JAK1/2 inhibitor. Complete regrowth was observed in a patient with ophiasis alopecia areata 9 months after starting oral baricitinib at 7 mg in the morning and 4 mg at night for treatment of concomitant CANDLE syndrome.92
Other JAK InhibitorsA study comparing delgocitinib ointment (pan-JAK inhibitor) 30 mg/g twice daily in vehicle for treatment of alopecia areata found no statistically significant differences in the decrease in the SALT score at 12 weeks (NCT02561585).93
The efficacy of ritlecitinib and brepocitinib was evaluated in a phase 2A trial of 142 patients with moderate to severe alopecia areata (NCT0297486894). After 6 weeks of treatment with brepocitinib and ritlecitinib, the mean difference in the SALT score over baseline was 12.44% and 19.36%, respectively, compared with 1.32% in the placebo group. Complete regrowth was observed in the responders after 24 weeks of treatment.
Another JAK1 inhibitor (SHR0302) is currently being investigated in adults with moderate to severe alopecia areata (NCT04346316).95
ConclusionsDysregulation of the JAK-STAT pathway could play a role in many dermatological diseases, including vitiligo and alopecia areata.
Oral and topical JAK inhibitors could be a promising option for treatment of vitiligo, although the results indicate that adjuvant treatment with NB-UVB or sunlight is necessary in order to obtain satisfactory results.
There is growing evidence that JAK inhibitors could prove effective in the treatment of alopecia areata, with an acceptable risk-benefit profile. Evaluation of tofacitinib, ruxolitinib, and baricitinib has yielded variable results. However, JAK inhibitors could be used as symptomatic treatment in alopecia areata, since the condition usually recurs when treatment is discontinued. Topical treatment with JAK inhibitors could prove to be an alternative in the case of localized disease, adverse effects with oral treatment, and children.
JAK inhibitors have also been used to treat other dermatologic diseases, such as psoriasis and atopic dermatitis. These conditions will be examined in depth in part 2 of the present review.
Conflicts of InterestC. Garcia-Melendo and X. Cubiró declare that they have no conflicts of interest. L. Puig has received fees from/participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, JS BIOCAD, Leo-Pharma, Lilly, Merck-Serono, MSD, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung-Bioepis, Sanofi, and UCB.
Please cite this article as: Garcia-Melendo C, Cubiró X, Puig L. Inhibidores de JAK: usos en dermatología. Parte 1: generalidades, aplicaciones en vitíligo y en alopecia areata. Actas Dermosifiliogr. 2021;112:503–515.