Dermatologists’ interest in the Janus-associated kinase (JAK)/signal transducers and activators of transcription (STAT) pathway has been growing as evidence builds to support its key role in the pathogenesis of inflammatory skin diseases. Because certain proinflammatory cytokines use the JAK/STAT pathway for signal transduction, it has become a promising therapeutic target in diseases where selective modulation of the immune system can be useful. We aim to review current knowledge of the JAK/STAT signaling pathway and its role in immune-mediated skin diseases. In the second part of the review we cover the efficacy and safety of oral and topical JAK inhibitors in the treatment of psoriasis, atopic dermatitis, and other skin diseases.
La vía de señalización de citocinas Janus cinasa/transductor de señal y activador de transcripción (JAK/STAT) es un área de interés emergente en dermatología, con evidencia creciente del papel clave en la patogénesis de las enfermedades inflamatorias cutáneas. Debido a que algunas citocinas proinflamatorias usan la vía JAK/STAT para la transducción de señales se convierte en una diana terapéutica prometedora para el tratamiento de dichas enfermedades al modular de forma selectiva el sistema inmune. El objetivo de esta revisión es conocer la vía de señalización JAK/STAT y su papel en distintas enfermedades dermatológicas inmunomediadas. En esta segunda parte, se revisará la eficacia y seguridad de los inhibidores de JAK –en formulación oral o tópica– para el tratamiento de la psoriasis, la dermatitis atópica y otras dermatosis.
Part 1 of this review provided a detailed description of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) intracellular signaling pathway. The potential role of JAK inhibitors in the treatment of various dermatologic diseases was highlighted, with emphasis on available evidence for vitiligo and alopecia areata.
Here, in Part 2, we review pathogenesis and the role of the JAK/STAT pathway in psoriasis and atopic dermatitis, as well as in other skin conditions such as hidradenitis, dermatomyositis, and graft-versus-host disease.
PsoriasisThe interleukin (IL) 23/IL-17 (IL-23/IL-17) axis is currently considered the main pathogenic pathway in psoriasis. However, several cytokines play a role in this disease, and some transmit their signal after binding to the corresponding receptors via the JAK/STAT pathway. These include interferon (IFN) γ, IFN-α, IL-2, IL-6, IL-12, IL-13, IL-19, IL-20, IL-21, IL-22, and IL-23. Tumor necrosis factor (TNF) α and other cytokines, such as IL-17, IL-8, and those of the IL-1 family (IL-1, IL-18, IL-36, and IL-38), do not directly activate the JAK/STAT pathway, although their activity can be suppressed indirectly through inhibition of the JAK/STAT pathway.1–3
JAK signaling has been shown to be overregulated, with increased expression of STAT1 and STAT3 in lesional psoriatic skin when compared with healthy skin.4–6
STAT1 is responsible for the transduction of type 1 IFN signals (α and β) and type 2 signals (γ) via a JAK1/JAK2–dependent mechanism, leading to production of multiple proinflammatory mediators and activation and maturation of dendritic cells with stimulation of type 1 helper T cells (TH1) and TH17.7,8
STAT3 is involved in the induction and differentiation of TH17 cells via activation of JAK2/TYK2 induced by IL-23.9 In addition, TH17 cells can produce IL-22, which is responsible for epidermal hyperplasia and production of antimicrobial peptides by keratinocytes.2,10 STAT3 also participates in the proliferation of keratinocytes via IL-6–induced activation of JAK1/JAK2 or JAK1/TYK211,12 and is indirectly activated by IL-17 through induction of IL-19 and/or IL-36 by keratinocytes.13
Oral TofacitinibTofacitinib (Xeljanz, Pfizer) mainly inhibits JAK1 and JAK3. It has been approved by the United States Food and Drug Administration (FDA) for the treatment of adults with psoriatic arthritis and for the treatment of moderate to severe rheumatoid arthritis at doses of 5mg twice daily.14 It was recently approved for the treatment of polyarticular juvenile idiopathic arthritis in children aged ≥2 years,15 as well as for treatment of ulcerative colitis at doses of 10mg twice daily for 8 weeks and subsequently at 5mg twice daily.14,16 Tofacitinib has proven effective in moderate to severe psoriasis in phase 2 and 3 trials.
In a study of 197 patients, treatment with tofacitinib 2mg, 5mg, and 15mg twice daily achieved a Psoriasis Area and Severity Index 75 (PASI75) response rate of 25%, 41%, and 67%, respectively, at 12 weeks, compared with 2% for placebo. PASI90 was achieved at 12 weeks by 22% of those who received tofacitinib.17
A phase 3 trial of 1106 patients with plaque psoriasis and PASI ≥12 revealed the noninferiority of tofacitinib 10mg/12h to subcutaneous etanercept 50mg at week 12, with a similar rate of adverse events in each group.18
Two phase 3 trials (OPT Pivotal 1 [901 patients] and OPT Pivotal 2 [960 patients]19), tofacitinib 10mg/12h was more efficacious than 5mg/12h from week 16 onward, with a greater PASI75 response rate between week 16 and week 28. Among the patients who reached PASI75 at week 16, the response was maintained at 52 weeks in 74.1% of the 5mg/12h group and 79.4% of the 10mg/12h group, and most continued to do so at 24 months.20 In addition, at 16 weeks of treatment, both doses led to an improvement in ungual psoriasis, pruritus (the difference was evident 1 day after initiation of treatment), and the Dermatology Life Quality Index. The improvement was maintained at week 52.21,22 Another phase 3 trial achieved an American College of Rheumatology (ACR) 20 response at week 16 in all patients with psoriatic arthritis treated with tofacitinib (5mg/12h or 10mg/12h) and an ACR50 or ACR70 in more than half. The responses were maintained at week 52.23
Most adverse effects were mild or moderate. In the tofacitinib groups, 12 patients had herpes zoster, although the most frequent adverse effect was nasopharyngitis.19 Two patients treated with tofacitinib 10mg every 12h in OPT Pivotal 1 had severe infections (appendicitis, pneumonia, and pyelonephritis), whereas 3 patients who received tofacitinib 5mg every 12h in OPT Pivotal 2 had severe infections (pneumonia, herpes zoster, erysipelas). Both studies reported an increase in cholesterol and creatine phosphokinase and reduced hemoglobin.
The adverse effects of tofacitinib are similar at 5mg and 10mg, with the most frequent being mild cytopenia, upper respiratory tract infections, headache, urinary tract infection, and diarrhea.17,19,20,24 In October 2015, the FDA refused to approve tofacitinib for treatment of moderate to severe plaque psoriasis, alleging that more studies were necessary on long-term safety.25
Topical TofacitinibTopical tofacitinib could be an alternative therapy in mild to moderate plaque psoriasis. It has a favorable safety profile, although the improvement reported in published studies is slight. In a phase 2a study of 71 patients, the authors reported promising results with tofacitinib ointment 2% every 12hours. The percentage change in the Target Plaque Severity Score at week 4 with respect to baseline was statistically significant with tofacitinib ointment compared with the vehicle (minimum mean square, −54.4% vs. −41.5%, respectively).26 A subsequent multicenter randomized study including 435 patients who received tofacitinib ointment 1% and 2% revealed greater efficacy than the vehicle for treatment of psoriasis at week 8 (Physician's Global Assessment [PGA] 0/1 in 18.6% of patients for tofacitinib 2% every 24hours and 22.5% for tofacitinib 2% every 12hours), although not at week 12. Reactions were observed at the application site in 8.1% of patients, although the highest incidence recorded for patients who received vehicle.27
Topical RuxolitinibThe JAK1/2 inhibitor ruxolitinib has been assessed as topical treatment for psoriasis. In a phase 2 clinical trial, 29 patients were randomized to receive ruxolitinib cream 0.5% or 1% once daily or 1.5% twice daily for 28 days, vehicle, or an active comparator (calcipotriene cream 0.005% or betamethasone dipropionate 0.05%). Treatment with ruxolitinib cream 1% and 1.5% was safe, well tolerated, and effective, with reduced plaque thickness, erythema, scaling, and lesion size compared with the vehicle (reduction of 53% in the treatment groups vs. 32% in the vehicle group). No significant differences in efficacy were found between ruxolitinib and the active comparators.28
Oral BaricitinibBaricitinib (Olumiant, Lilly) is a JAK1/2 inhibitor that has been approved by the FDA29 and the European Medicines Agency (EMA)30 for the treatment of moderate to severe rheumatoid arthritis at recommended doses of 4mg/d. It was recently approved for the treatment of moderate to severe atopic dermatitis.30
Baricitinib was studied in a phase 2b trial for treatment of moderate to severe plaque psoriasis in 271 patients. The PASI75 at week 12 was significantly higher for the groups treated with 8mg and 10mg once daily than for placebo (42.9%, 54.1%, and 16.1%, respectively). The most common adverse effects were infection, with an incidence rate of 26.5% in the placebo group and 21.5% in the baricitinib groups, the most common being nasopharyngitis.31
Oral AbrocitinibAbrocitinib (Pfizer) is a JAK1 inhibitor that was assessed for the treatment of moderate to severe psoriasis in a phase 2 trial lasting 12 weeks, in which 59 patients were randomly assigned to receive 200mg/24h, 400mg/24h, 200mg/12h, or placebo for 4 weeks. At week 4, the percentage of patients who achieved PASI75 was 17% for the placebo and 200mg/24h groups, 50% for the 400mg/24h group, and 60% for the 200mg/12h group. More laboratory abnormalities (low neutrophil, platelet, and reticulocyte counts) were observed in the 200mg/12h group, although no associated bleeding or severe infections were recorded.32
Oral SolcitinibA dose-dependent improvement in psoriasis was recorded with solcitinib (GlaxoSmithKline), a JAK1 inhibitor that was assessed in a phase 2 trial. The study population comprised 60 patients, of whom 13% achieved PASI75 at 100mg/d, 25% at 200mg/d, 57% at 400mg/d, and 0% with placebo.33
Oral Itacitinib AdipateThe JAK1 inhibitor itacitinib adipate (Incyte Corporation) led to a significant improvement in patients with moderate to severe psoriasis in a phase 2 trial including 50 patients and lasting 12 weeks. At 1 month, PASI75 was achieved by 0%, 11.1%, 0%, 22.2%, and 27.7% in the placebo, 100mg/24h, 200mg/24h, 200mg/12h, and 600mg/24h groups, respectively. The only significant differences with placebo were observed in the itacitinib 600mg/d group.34
Oral PeficitinibPeficitinib (Smyraf, Astellas Pharma Inc.) is a pan-JAK inhibitor that has been approved in Japan for treatment of rheumatoid arthritis35 that proved efficacious in a phase 2 trial including 124 patients with moderate to severe plaque psoriasis. Patients received active treatment (10mg, 25mg, 60mg, 100mg twice daily or 50mg once daily) or placebo. The mean improvement in PASI and body surface area with respect to baseline was significantly better in all treatment groups than in the placebo group at 6 weeks. The difference was dose-dependent.36
TYK2 InhibitorsCurrent studies focus on TYK2 inhibitors for the treatment of moderate to severe psoriasis.
The percentage of patients who reached PASI75 at 12 weeks in a phase 2 trial (267 patients) was significantly greater with deucravacitinib (a TYK2 inhibitor, Bristol-Myers Squibb) than with placebo (39% at 3mg/24h, 69% at 3mg/12h, 67% at 6mg/12h, and 75% at 12mg/24h vs. 7% in the placebo group). The PASI90 response rate was 43% at 12 weeks, and the PASI100 response rate was 25%.37 The ongoing phase 3 trials with this drug (NCT04036435,38 NCT0392442739) include one in which it is compared with apremilast (NCT0361175140).
Brepocitinib (Pfizer) is a potent TYK2/JAK1 inhibitor that is being evaluated for treatment of psoriasis. In a phase 1 trial, 30 patients with moderate to severe plaque psoriasis received 30mg or 100mg orally or placebo once daily for 28 days. A PGA 0/1 response was achieved in 57.1%, 100%, and 0% of cases, respectively.41 Topical application of brepocitinib is currently being tested in a phase 2b trial in patients with mild to moderate psoriasis (NCT0385048342).
Finally, another TYK2 inhibitor (PF-06826647, Pfizer) is being investigated for moderate to severe psoriasis in a phase 2 trial (NCT0389537243).
Atopic DermatitisAtopic dermatitis is an inflammatory skin disease with a TH2-polarized immune response in its acute phase. The JAK/STAT pathway plays a key role in the dysregulation of the immune response in atopic dermatitis, including overregulation of TH2, activation of eosinophils, suppression of regulatory T cells, and maturation of B cells, with differentiation to plasma cells and secretion of immunoglobulin (Ig) E, which binds to cutaneous mastocytes and leads to release of histamine. Similarly, the TH2 response leads to release of proinflammatory cytokines and proangiogenic factors by epidermal cells.
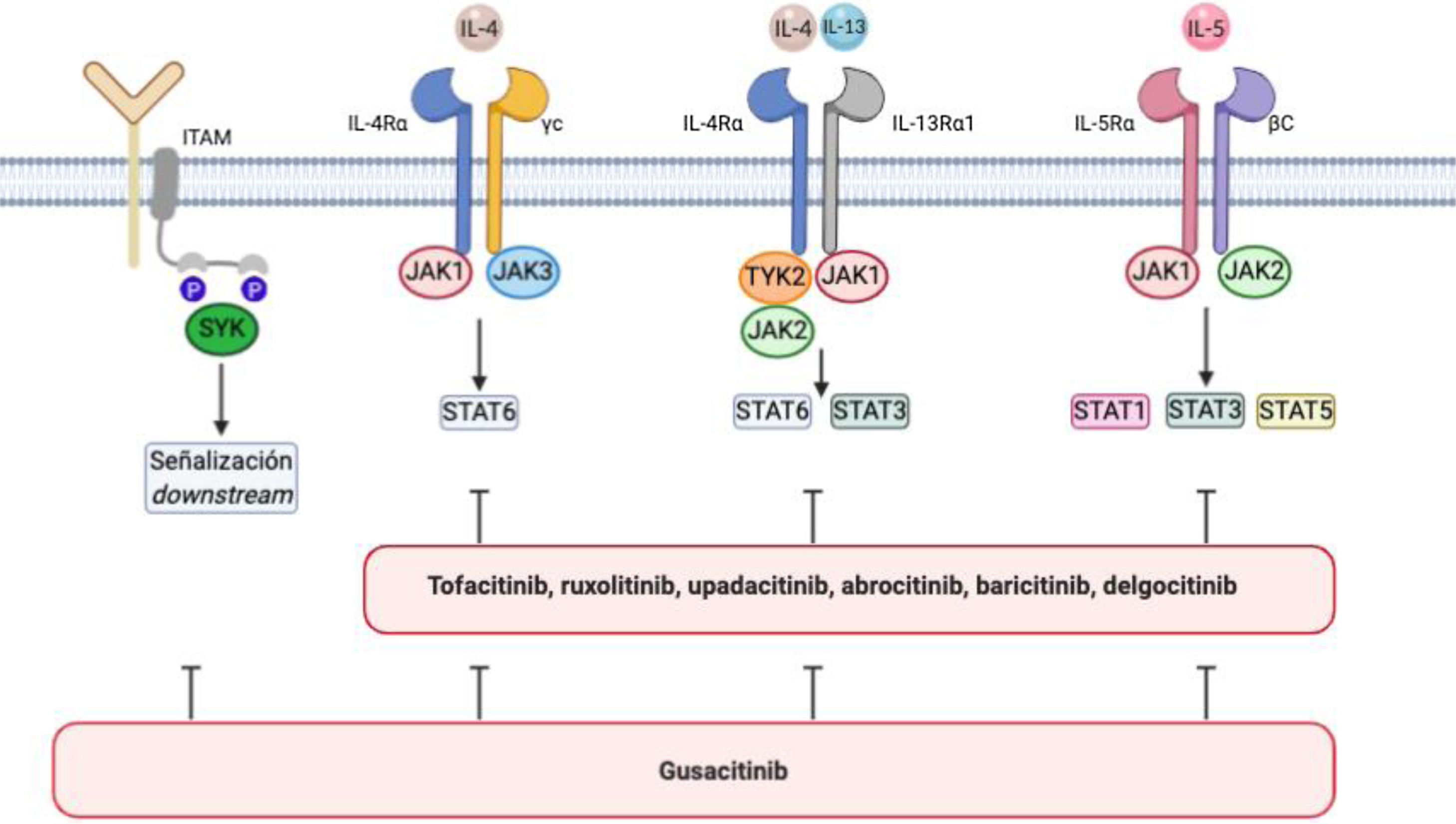
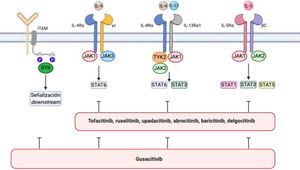
Atopic dermatitis is associated with an increase in signaling via JAK1, JAK2, JAK3, and TYK244 (Fig. 1). JAK1, JAK3, and STAT6 are components of IL-4 signaling that are critical for TH2 cell differentiation45–47 and production of IL-4, IL-5, IL-10, and IL-13. STAT6 regulates the genes involved in TH2 cells and B-cell differentiation, switching from IgG to IgE, and production of class II major histocompatibility complex molecules. Various STAT6 polymorphisms have been associated with greater susceptibility to atopic dermatitis and high IgE levels.48
IL-4/IL-13/JAK/STAT signaling pathway and SYK pathway in atopic dermatitis.
The figure shows various JAK inhibitors and dual JAK/SYK inhibitors in atopic dermatitis. The biological functions of IL-4 and IL-13 are mediated by their binding to the subunits IL-4Rα and IL-13Rα1 of the corresponding receptors. IL-4 binds to a type 1 receptor that includes IL-4Rα and the γ chain of the cytokine receptor or a type 2 receptor configured by IL-4Rα and IL-13R α1. The latter is the main IL-13 receptor.
IL-5 produced as a result of polarization of type 2 helper T cells binds to the IL-5Rα subunit of its receptor to form a complex with a shared signaling subunit, the β chain, and induce phosphorylation of JAK1/JAK2 and activation of STAT1, STAT3, and STAT5.
Via its SH2 domains, SYK binds to diphosphorylated immunoreceptor tyrosine-based activation motifs (ITAM) in the cytoplasm of various immune receptors, such as T-, B-, NK-cell receptors or the various receptors for the constant fraction of immunoglobulin (Fc) in neutrophils, mastocytes, macrophages dendritic cells, and other strains of the immune system. This interaction results in the activation of other proteins that transmit downstream signals.
Figure generated with the assistance of Biorender.com.
The IL-4/IL-13/JAK/STAT pathway49 is important for the integrity of the skin barrier. Suppression by nonselective JAK inhibitors of STAT3 activation induced by IL-4/IL-13 has been shown to improve barrier function, thus promoting production of filaggrin and loricrin.50 Furthermore, IL-4 and IL-13 play a role in pruritus through their interaction with IL-4Rα and JAK1 in sensory neurons.51
Factors other than IL-4 are important for differentiation of TH2. Thymic stromal lymphopoietin (TSLP) promotes TH2 cell differentiation, activates natural killer cells and basophils, and affects maturation of B cells. TSLP binds to a heterodimer composed of a TSLP receptor and an IL-7α receptor and induces phosphorylation of JAK1 and JAK2, leading to activation of STAT1, STAT3, and STAT5.52
The spleen tyrosine kinase (SYK) pathway is also involved in the pathogenesis of atopic dermatitis. SYK is a cytoplasmic tyrosine kinase that is involved in signaling of proinflammatory cytokines and production of CCL20, which attracts TH17 cells to the skin53,54 (Fig. 1). Inhibition of SYK suppresses this activation and has an anti-inflammatory effect, which may be synergetic with concomitant inhibition of JAK. Favorable results have been reported for some dual inhibitors used to treat atopic dermatitis.
Oral TofacitinibAn open-label study of 6 patients treated with tofacitinib 5mg once or twice daily, in addition to topical treatment, revealed a 36.6% reduction in the Scoring Atopic Dermatitis (SCORAD) score at weeks 8 to 12, and of 12% at week 29, with no remarkable adverse effects.55
Topical TofacitinibApplication of topical tofacitinib 2% (20mg/g) every 12h in 69 patients with mild to moderate atopic dermatitis for 4 weeks led to an 81.7% reduction in the Eczema Area and Severity Index (EASI) score after 4 weeks, compared with 29.9% in patients receiving placebo. The Investigator Global Assessment (IGA), body surface area, and pruritus also improved from the first week of treatment.56
Oral BaricitinibBaricitinib (Olumiant, Lilly) has been evaluated in a phase 2 trial and in 2 phase 3 trials for treatment of moderate to severe atopic dermatitis.
Baricitinib was used concomitantly with topical triamcinolone 0.1% for 16 weeks in a phase 2 trial with 124 patients.57 Severity of atopic dermatitis improved significantly in those who received 2mg and 4mg compared with placebo, with a rapid improvement in pruritis during the first week and in health-related quality of life. In the group treated with baricitinib 4mg, 61% of patients achieved an EASI50 response at 16 weeks compared with 37% in the placebo group.
In 2 phase 3 studies in adults (BREEZE-AD1 [624 patients] and BREEZE-AD2 [615 patients), baricitinib improved the severity of atopic dermatitis at 16 weeks and led to a rapid reduction in pruritus from the first week. IGA 0/1 was reached in BREEZE-AD1 and BREEZE-AD2 at week 16, respectively, in 4.8% and 4.5% of patients in the placebo group and in 11.8% and 8.8% of the groups treated with baricitinib 1mg, 11.4% and 10.6% in those treated with baricitinib 2mg, and 16.8% and 13.8% of those treated with baricitinib 4mg.58 The most common adverse events were nasopharyngitis, headache, and increased creatine phosphokinase. Long-term safety is currently being evaluated (NCT0333443559).
Baricitinib was recently approved by the European Medicines Agency for treatment of moderate to severe atopic dermatitis in adult patients who were candidates for systemic treatment, with a recommended dose of 4mg/d.30
Oral UpadacitinibUpadacitinib (Rinvoq, AbbVie) is a JAK1 inhibitor that has been approved for the treatment of rheumatoid arthritis.60 The efficacy and safety profile of the drug in adults with moderate to severe atopic dermatitis was evaluated in a phase 2b clinical trial. A total of 167 patients were randomly assigned to receive 3 different doses of upadacitinib (7.5mg, 15mg, and 30mg daily) or placebo. The mean percentage improvement in the EASI at week 16 over baseline was higher than for placebo at all the doses of upadacitinib (39% for 7.5mg, 62% for 15mg, 74% for 30mg vs. 23% in the placebo group). An EASI100 response was observed in 24% of the patients treated with 30mg/d, but not in those treated with placebo.
Results were recently published from 2 phase 3 trials, Measure Up 1 and Measure Up 2, which evaluate the efficacy and safety of upadacitinib for treatment of moderate to severe atopic dermatitis in adults and adolescents (NCT0356929361 and NCT0360742262). EASI75 was achieved at 16 weeks by 70% and 60% of the patients who received upadacitinib 15mg (n=281 [Measure Up 1] and n=276 [Measure Up 2]), by 80% and 73% of those who received upadacitinib 30mg (n=285 [Measure Up 1] and n=282 [Measure Up 2]), and by only 16% and 13% in the placebo group (n=281 [Measure Up 1] and n=278 [Measure Up 2]). IGA 0/1 was achieved by 48% and 39% of patients treated with upadacitinib 15mg and 62% and 52% of those treated with upadacitinib 30mg, compared with 8% and 5% of the placebo groups. Both doses led to an early reduction in pruritus, which remained unchanged at 16 weeks. The most common adverse effects were acne, nasopharyngitis, upper respiratory tract infection, and headache.63,64
The safety and pharmacokinetics of various doses of upadacitinib are being evaluated in children with severe atopic dermatitis (NCT0364660465), and a phase 3 trial comparing upadacitinib with dupilimab in adults is currently underway (NCT0373839766).
Oral AbrocitinibAbrocitinib may be effective and well-tolerated in patients with moderate to severe atopic dermatitis.
A 12-week placebo-controlled phase 2b study included 269 adults treated with 4 daily doses of abrocitinib (10, 30, 100, and 200mg). The EASI improved significantly compared with placebo only for 100 and 200mg/d (reduction of 59% and 82.6% respectively vs. 35.2% in the placebo group), with a reduction in pruritus as early as day 2 of the study.67
In a recent phase 3 trial, 391 adolescents and adults were treated with abrocitinib 200mg or 100mg or placebo over 12 weeks. A greater percentage of patients in the abrocitinib 200mg and 100mg groups vs. placebo reached IGA 0/1 (38.1% and 28.4% vs. 9.1%, respectively), EASI75 (61% and 44.5% vs. 10.4%, respectively), and EASI90 (37.7% and 23.9% vs. 3.9%, respectively). Adverse effects were recorded in 65.8% of patients who received 200mg, in 62.7% of the group that received 100mg, and in 53.8% of the placebo group. The most frequent were nausea in the 200-mg group, nasopharyngitis in the 100-mg group, and atopic dermatitis in the placebo group. Severe adverse effects were observed in the abrocitinib 200mg, 100mg, and placebo groups, affecting 1.3%, 3.2%, and 1.3% of patients, respectively. The effects considered to be associated with treatment included 1 case of herpangina and 1 case of pneumonia in the abrocitinib 100-mg group. Two patients in the abrocitinib 200-mg group developed herpes zoster, and 2 patients in the abrocitinib 100-mg group developed eczema herpeticum. Furthermore, in the group treated with 200mg, the platelet count decreased in 2 patients, and thrombocytopenia was detected in 5 patients.68
The long-term safety and efficacy profile of abrocitinib is being studied in adolescents (NCT03796676,69 NCT03627767,70 NCT03422822,71). Ongoing studies are comparing the efficacy of oral abrocitinib with subcutaneous dupilumab in adults with atopic dermatitis (NCT04345367,72 NCT0372047073).
Oral GusacitinibGusacitinib (Asana BioSciences) is a dual inhibitor of the JAK and SYK pathways that is currently being trialed as oral medication. In a phase 1b trial involving 36 patients, EASI50 was achieved and pruritis decreased in almost all patients at 4 weeks of treatment with 20, 40, or 80mg. An EASI75 response was achieved in 63% of patients treated with 40mg and in 50% of those treated with 80mg/d compared with 22% in the placebo group. The improvement in the EASI response was associated with a decrease in the levels of the cutaneous biomarkers TH2 and TH22.74
Results are pending from a phase 2b placebo-controlled trial to evaluate the efficacy and safety of ASN002 in 220 patients with moderate to severe atopic dermatitis (NCT0353195775).
Topical RuxolitinibIn a phase 2b trial,76 307 patients with mild to moderate atopic dermatitis were randomized to receive ruxolitinib cream 1.5%/12h, 1.5%/24h, 0.5%/24h, or 0.15%/24h for 8 weeks, vehicle, or acetonide triamcinolone 0.1% cream every 12hours for 4 weeks. A therapeutic benefit was recorded for all of the ruxolitinib regimens at week 4, although the regimen that led to the greatest change in EASI was ruxolitinib 1.5%/12h (71.6% vs. 15.5% for vehicle). The improvement in EASI was greater in patients treated with ruxolitinib 1.5% every 12hours or every 24hours than in those treated with triamcinolone 0.1%, although the differences were not statistically significant. Furthermore, ruxolitinib managed to improve pruritis quickly. The improvement was maintained, the drug was well tolerated, and no relevant reactions were observed at the application site.
A phase 1 trial is currently evaluating the safety and pharmacokinetics of topical ruxolitinib in pediatric patients (NCT0325764477), and other phase 3 trials include adolescents (NCT03745638,78 NCT0374565179).
Topical DelgocitinibThe JAK1, JAK2, and JAK3 inhibitor delgocitinib (Corectim, Japan Tobacco/LEO Pharma) was developed for topical treatment of atopic dermatitis.80 Phase 1 and 2 studies81,82 proved the concept of therapeutic efficacy with respect to severity and pruritus. Pruritus improved 1 day after initiating delgocitinib, probably owing to the inhibition of IL-31.
Results were recently published from a study that evaluated the safety and efficacy profile of delgocitinib 0.5% ointment in 158 Japanese patients aged ≥16 years with moderate to severe atopic dermatitis. The mean percentage change in EASI after 4 weeks was −44.3% in the delgocitinib group and 1.7% in the vehicle group; the results remained unchanged at 24 weeks. Various adverse effects were recorded, although these were mainly mild, affecting 4.7% of patients in the delgocitinib group and 1.9% in the vehicle group. The most common adverse effect was nasopharyngitis, followed by Kaposi varicelliform eruption and acne.83
Topical delgocitinib 0.5% (Corectim) has been approved in Japan for treatment of atopic dermatitis.84 Ongoing studies are evaluating various concentrations (NCT0372572285) and include pediatric patients (NCT0382690186).
Other OptionsAn ongoing study is evaluating the safety and efficacy profile of the JAK1 inhibitor SHR0302 (NCT0416289987) for treatment of patients with moderate to severe atopic dermatitis. Results are pending from another study with topical brepocitinib for treatment of mild to moderate atopic dermatitis (NCT03903822).88
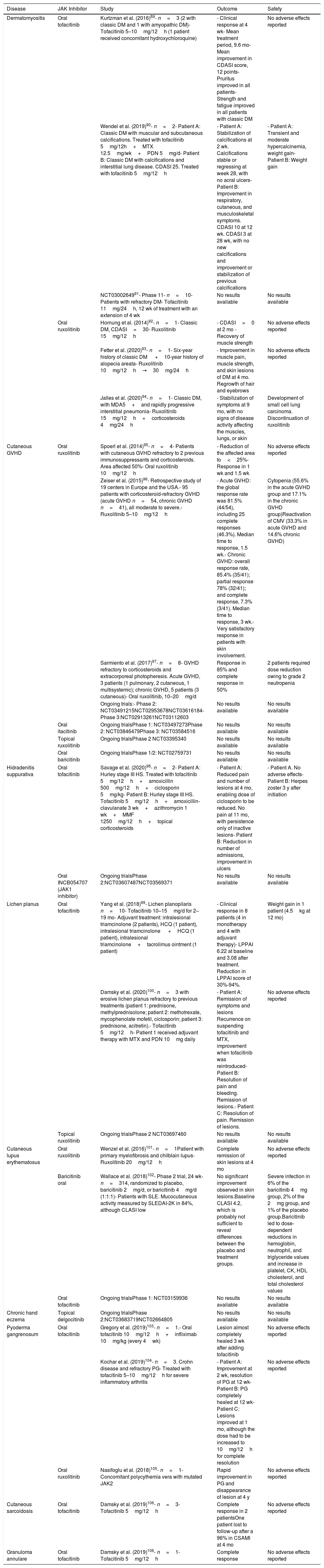
Other Applications in DermatologyJAK inhibitors were recently used for the treatment of other treatment-refractory dermatologic conditions in which activation of the JAK/STAT pathway plays a key role (Table 1).
Use of JAK Inhibitors in Other Skin Conditionsa
| Disease | JAK Inhibitor | Study | Outcome | Safety |
|---|---|---|---|---|
| Dermatomyositis | Oral tofacitinib | Kurtzman et al. (2016)89- n=3 (2 with classic DM and 1 with amyopathic DM)- Tofacitinib 5–10mg/12h (1 patient received concomitant hydroxychloroquine) | - Clinical response at 4 wk- Mean treatment period, 9.6 mo- Mean improvement in CDASI score, 12 points- Pruritus improved in all patients- Strength and fatigue improved in all patients with classic DM | No adverse effects reported |
| Wendel et al. (2019)90- n=2- Patient A: Classic DM with muscular and subcutaneous calcifications. Treated with tofacitinib 5mg/12h+MTX 12.5mg/wk+PDN 5mg/d- Patient B: Classic DM with calcifications and interstitial lung disease. CDASI 25. Treated with tofacitinib 5mg/12h | - Patient A: Stabilization of calcifications at 2 wk. Calcifications stable or regressing at week 28, with no acral ulcers- Patient B: Improvement in respiratory, cutaneous, and musculoskeletal symptoms. CDASI 10 at 12 wk. CDASI 3 at 28 wk, with no new calcifications and improvement or stabilization of previous calcifications | - Patient A: Transient and moderate hypercalcinemia, weight gain- Patient B: Weight gain | ||
| NCT0300264991- Phase 11- n=10- Patients with refractory DM- Tofacitinib 11mg/24h, 12 wk of treatment with an extension of 4 wk | No results available | No results available | ||
| Oral ruxolitinib | Hornung et al. (2014)92- n=1- Classic DM, CDASI=30- Ruxolitinib 15mg/12h | - CDASI=0 at 2 mo -Recovery of muscle strength | No adverse effects reported | |
| Fetter et al. (2020)93- n=1- Six-year history of classic DM +10-year history of alopecia areata- Ruxolitinib 10mg/12h→30mg/24h | - Improvement in muscle pain, muscle strength, and skin lesions of DM at 4 mo. Regrowth of hair and eyebrows | No adverse effects reported | ||
| Jalles et al. (2020)94- n=1- Classic DM, with MDA5+and rapidly progressive interstitial pneumonia- Ruxolitinib 15mg/12h+corticosteroids 4mg/24h | - Stabilization of symptoms at 9 mo, with no signs of disease activity affecting the muscles, lungs, or skin | Development of small cell lung carcinoma. Discontinuation of ruxolitinib | ||
| Cutaneous GVHD | Oral ruxolitinib | Spoerl et al. (2014)95- n=4- Patients with cutaneous GVHD refractory to 2 previous immunosuppressants and corticosteroids. Area affected 50%- Oral ruxolitinib 10mg/12h | - Reduction of the affected area to<25%- Response in 1 wk and 1.5 wk | No adverse effects reported |
| Zeiser et al. (2015)96- Retrospective study of 19 centers in Europe and the USA.- 95 patients with corticosteroid-refractory GVHD (acute GVHD n=54, chronic GVHD n=41), all moderate to severe.- Ruxolitinib 5–10mg/12h | - Acute GVHD: the global response rate was 81.5% (44/54), including 25 complete responses (46.3%). Median time to response, 1.5 wk.- Chronic GVHD: overall response rate, 85.4% (35/41); partial response 78% (32/41); and complete response, 7.3% (3/41). Median time to response, 3 wk.- Very satisfactory response in patients with skin involvement. | Cytopenia (55.6% in the acute GVHD group and 17.1% in the chronic GVHD group)Reactivation of CMV (33.3% in acute GVHD and 14.6% chronic GVHD) | ||
| Sarmiento et al. (2017)97- n=8- GVHD refractory to corticosteroids and extracorporeal photopheresis. Acute GVHD, 3 patients (1 pulmonary, 2 cutaneous, 1 multisystemic); chronic GVHD, 5 patients (3 cutaneous)- Oral ruxolitinib, 10–20mg/d | Response in 85% and complete response in 50% | 2 patients required dose reduction owing to grade 2 neutropenia | ||
| Ongoing trials:- Phase 2: NCT03491215NCT02953678NCT03616184- Phase 3:NCT02913261NCT03112603 | No results available | No results available | ||
| Oral itacitinib | Ongoing trialsPhase 1: NCT03497273Phase 2: NCT03846479Phase 3: NCT03584516 | No results available | No results available | |
| Topical ruxolitinib | Ongoing trialsPhase 2 NCT03395340 | No results available | No results available | |
| Oral baricitinib | Ongoing trialsPhase 1/2: NCT02759731 | No results available | No results available | |
| Hidradenitis suppurativa | Oral tofacitinib | Savage et al. (2020)98- n=2- Patient A: Hurley stage III HS. Treated with tofacitinib 5mg/12h+amoxicillin 500mg/12h+ciclosporin 5mg/kg- Patient B: Hurley stage III HS. Tofacitinib 5mg/12h+amoxicillin-clavulanate 3 wk+azithromycin 1 wk+MMF 1250mg/12h+topical corticosteroids | - Patient A: Reduced pain and number of lesions at 4 mo, enabling dose of ciclosporin to be reduced. No pain at 11 mo, with persistence only of inactive lesions- Patient B: Reduction in number of admissions, improvement in ulcers | - Patient A. No adverse effects- Patient B: Herpes zoster 3 y after initiation |
| Oral INCB054707 (JAK1 inhibitor) | Ongoing trialsPhase 2:NCT03607487NCT03569371 | No results available | No results available | |
| Lichen planus | Oral tofacitinib | Yang et al. (2018)99- Lichen planopilaris n=10- Tofacitinib 10–15mg/d for 2–19 mo- Adjuvant treatment: intralesional triamcinolone (2 patients), HCQ (1 patient), intralesional triamcinolone +HCQ (1 patient), intralesional triamcinolone+tacrolimus ointment (1 patient) | - Clinical response in 8 patients (4 in monotherapy and 4 with adjuvant therapy)- LPPAI 6.22 at baseline and 3.08 after treatment. Reduction in LPPAI score of 30%-94%. | Weight gain in 1 patient (4.5kg at 12 mo) |
| Damsky et al. (2020)100- n=3 with erosive lichen planus refractory to previous treatments (patient 1: prednisone, methylprednisolone; patient 2: methotrexate, mycophenolate mofetil, ciclosporin; patient 3: prednisone, acitretin).- Tofacitinib 5mg/12h- Patient 1 received adjuvant therapy with MTX and PDN 10mg daily | - Patient A: Remission of symptoms and lesions Recurrence on suspending tofacitinib and MTX, improvement when tofacitinib was reintroduced- Patient B: Resolution of pain and bleeding. Remission of lesions.- Patient C: Resolution of pain. Remission of lesions. | No adverse effects reported | ||
| Topical ruxolitinib | Ongoing trialsPhase 2 NCT03697460 | No results available | No results available | |
| Cutaneous lupus erythematosus | Oral ruxolitinib | Wenzel et al. (2016)101- n=1Patient with primary myelofibrosis and chilblain lupus-Ruxolitinib 20mg/12h | Complete remission of skin lesions at 4 mo | No adverse effects reported |
| Baricitinib oral | Wallace et al. (2018)102- Phase 2 trial, 24 wk- n=314, randomized to placebo, baricitinib 2mg/d, or baricitinib 4mg/d (1:1:1)- Patients with SLE. Mucocutaneous activity measured by SLEDAI-2K in 84%, although CLASI low | No significant improvement observed in skin lesions.Baseline CLASI 4.2, which is probably not sufficient to reveal differences between the placebo and treatment groups. | Severe infection in 6% of the baricitinib 4mg group, 2% of the 2mg group, and 1% of the placebo group.Baricitinib led to dose-dependent reductions in hemoglobin, neutrophil, and triglyceride values and increase in platelet, CK, HDL cholesterol, and total cholesterol values | |
| Oral tofacitinib | Ongoing trialsPhase 1: NCT03159936 | No results available | No results available | |
| Chronic hand eczema | Topical delgocitinib | Ongoing trialsPhase 2:NCT03683719NCT02664805 | No results available | No results available |
| Pyoderma gangrenosum | Oral tofacitinib | Gregory et al. (2019)103- n=1.- Oral tofacitinib 10mg/12h+infliximab 10mg/kg (every 4wk) | Lesion almost completely healed 3 wk after adding tofacitinib | No adverse effects reported |
| Kochar et al. (2019)104- n=3. Crohn disease and refractory PG- Treated with tofacitinib 5–10mg/12h for severe inflammatory arthritis | - Patient A: Improvement at 2 wk, resolution of PG at 12 wk- Patient B: PG completely healed at 12 wk- Patient C: Lesions improved at 1 mo, although the dose had to be increased to 10mg/12h for complete resolution | No adverse effects reported | ||
| Oral ruxolitinib | Nasifoglu et al. (2018)105- n=1- Concomitant polycythemia vera with mutated JAK2 | Rapid improvement in PG and disappearance of lesion at 4 y | No adverse effects reported | |
| Cutaneous sarcoidosis | Oral tofacitinib | Damsky et al. (2019)106- n=3- Tofacitinib 5mg/12h | Complete response in 2 patientsOne patient lost to follow-up after a 96% in CSAMI at 4 mo | No adverse effects reported |
| Granuloma annulare | Oral tofacitinib | Damsky et al. (2019)106- n=1- Tofacitinib 5mg/12h | Complete response | No adverse effects reported |
Ongoing trials: information from ClinicalTrials.gov.107
Abbreviations. CDASI, Cutaneous Dermatomyositis Disease Area and Severity Index; CK, creatine kinase; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; CMV, cytomegalovirus; CSAMI, cutaneous sarcoidosis activity and morphology instrument; DM, dermatomyositis; GVHD, graft-versus-host disease; HCQ, hydroxychloroquine; HS, hidradenitis suppurativa; SLE, systemic lupus erythematosus; LPPAI, Lichen Planopilaris Activity Index; MMF, mycophenolate mofetil; MTX, methotrexate; PDN, prednisone; PG, pyoderma gangrenosum; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2000.
Dysregulation of the JAK/STAT pathway could intervene in the pathogenesis of many dermatologic diseases, including vitiligo, alopecia areata, psoriasis, and atopic dermatitis. JAK inhibitors, which have an acceptable safety profile, could be used for the simultaneous treatment of pathogenically similar inflammatory skin diseases with common signaling pathways.
In the case of moderate to severe psoriasis, tofacitinib has been evaluated in phase 1, 2, and 3 trials, all of which show it to be efficacious, especially at 10mg/12h, although the drug has not been approved by regulatory committees. Recent data suggest that TYK2 inhibitors could show promising results for the treatment of psoriasis. One such drug, deucravacitinib, has shown maximum efficacy for oral treatment of psoriasis. Current therapeutic options in atopic dermatitis are limited, although oral and topical JAK inhibitors have yielded promising results, with improvement in the severity of atopic dermatitis and a rapid and marked improvement in pruritus and quality of life. Baricitinib was recently approved for the treatment of moderate to severe atopic dermatitis in adults.
In the future, JAK inhibitors could prove to be a real alternative therapy for some inflammatory skin diseases. Selective inhibitors have a superior safety and efficacy profile, whereas nonselective inhibitors are being developed mainly for topical use. The inhibition of multiple signals, especially the response to interferon, could increase susceptibility to infection and viral reactivation. While the safety and efficacy profile seems favorable for the moment, more studies are necessary to determine the doses that will optimize efficacy, cost-effectiveness, and safety of this drug family for potential use in skin conditions in the long term.
Conflicts of InterestC. Garcia-Melendo declares that she has no conflicts of interest.
X. Cubiró has received fees from Novartis, Leo-Pharma, and Almirall.
L. Puig has received fees and/or has participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, JS BIOCAD, Leo-Pharma, Lilly, Merck-Serono, MSD, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung-Bioepis, Sanofi, and UCB.
Please cite this article as: Garcia-Melendo C, Cubiró X, Puig L. Inhibidores de JAK: usos en dermatología. Parte 2: aplicaciones en psoriasis, dermatitis atópica y otras dermatosis. Actas Dermosifiliogr. 2021;112:586–600.