Recessive dystrophic epidermolysis bullosa (RDEB) is among the most serious rare skin diseases. It is also the rare skin disease for which most effort has been expended in developing advanced therapeutic interventions. RDEB is caused by collagen VII deficiency resulting from COL7A1 mutations. Therapeutic approaches seek to replenish collagen VII and thus restore dermal-epidermal adhesion. Therapeutic options under development include protein therapy and different cell-based and gene-based therapies. In addition to treating skin defects, some of these therapies may also target internal mucosa. In the coming years, these novel therapeutic approaches should substantially improve the quality of life of patients with RDEB.
La epidermólisis bullosa distrófica recesiva (EBDR) es una de las enfermedades raras (ER) de la piel más graves y que mayor interés ha suscitado en cuanto al desarrollo de estrategias avanzadas de intervención terapéutica. La EBDR es debida a la deficiencia de colágeno VII como consecuencia de mutaciones en el gen COL7A1, y los distintos abordajes terapéuticos buscan la reposición de colágeno VII para restituir la adhesión dermo-epidérmica. La variedad de terapias en evaluación incluyen tanto la proteica como diversas estrategias celulares y génicas. Algunas de estas estrategias tienen un potencial terapéutico que va más allá del defecto cutáneo, pudiendo corregir el problema también a nivel de las mucosas internas. En los próximos años se espera que estos nuevos abordajes brinden una mejora sustancial en la calidad de vida de los pacientes con EBDR.
Rare genetic skin diseases, or genodermatoses, are an important part of clinical and pathologic practice in dermatology. The unraveling of the genetic basis of approximately 400 such diseases, most of which are monogenic, has paved the way for molecular diagnoses, more accurate disease classification, and a greater understanding of the pathogenic mechanisms involved. Despite this new body of knowledge, however, treatment is still limited to palliative care in the vast majority of cases.
Genetic skin diseases comprise epithelial adhesion, keratinization, pigmentation, DNA repair, and connective tissue disorders and ectodermal dysplasias. In this review, we will focus on epithelial adhesion disorders, which include epidermolysis bullosa (EB). EB comprises a group of heritable skin diseases characterized by fragile skin and mucous membranes and the formation of blisters in response to mechanical trauma or not. EB has a wide spectrum of phenotypes, ranging from mild features due to subtle molecular defects to severe cutaneous and extracutaneous manifestations due to severely compromised dermal-epidermal adhesion. The estimated prevalence of EB is between 8 and 25 cases per million population.1 Patients with EB need special care aimed at treating both the primary manifestations of the disease and their potential complications, which, in some cases, can be fatal.
Thanks to the identification of mutations in genes encoding proteins at the dermal-epidermal junction in recent years—18 mutations have been identified to date—we have gradually gained a better understanding of modes of inheritance and of genotype-phenotype correlations in the different forms of EB. These discoveries have also helped to improve the classification of the various types of EB. For an updated discussion of the classification of EB, we refer readers to the recent review by Fine et al.2 One of the most remarkable advances in the field is the variety of innovative treatment strategies that has come with the knowledge gained of the molecular basis of EB.3 In this review, we focus on novel treatment approaches for the recessive dystrophic form of EB (RDEB).
Molecular Characteristics of RDEBBoth RDEB and dominant dystrophic EB are caused by mutations in the COL7A gene encoding type VII collagen (C7). This protein is the main component of the anchoring fibers that link type I dermal collagen to proteins such as laminin-332 (formerly laminin-5) in the basement membrane. C7 acts as a dermal-epidermal adhesive not only in the skin but also in the mucous membranes and esophagus. These anchoring fibers are observable by electron microscopy and can also be visualized as a continuous band in the basement membrane by immunofluorescence staining with antibodies specific to C7 in biopsy specimens from normal skin. The absence or marked reduction of C7 is a hallmark finding in RBEB and is used to establish an initial molecular diagnosis, which must then be confirmed genetically. Restoration of C7 therefore lies at the root of all current treatments for RDEB, although, as will be seen, the sources of the protein vary.
C7 is synthesized primarily by epidermal (or mucosal) keratinocytes and to a lesser extent (an estimated 30% less) by dermal fibroblasts. Once synthesized, the protein is secreted and migrates with ease to its intended target site in the basement membrane. This concept is important when it comes to designing gene- or cell-based therapies, as it is not strictly necessary for all cells to produce C7, but rather to ensure a sufficient supply of this protein to guarantee dermal-epidermal adhesion.
Our group recently described the spectrum of COL7A1 mutations in a cohort of Spanish patients with RDEB and dominant dystrophic EB.4 Prior to the publication of this study, it was thought that each mutation might be unique to the family in which it occurred, with little or no recurrence. This was partly because multiple mutations had been described along the nucleotide sequence of the gene. However, to our surprise, we found that the c.6527insC mutation in exon 80 of COL7A1 was present in a high proportion of cases and accounted for almost 50% of the alleles identified.4 Subsequent investigations confirmed a founder effect for this mutation in the Spanish population.5 This nucleotide insertion alters the reading frame of messenger RNA (mRNA), resulting in a premature stop codon. The effect in individuals homozygous for the mutation is total absence of C7 at the dermal-epidermal junction, resulting in the severe mucocutaneous fragility observed. The large number of patients carrying this homozygous mutation means that targeted therapies could be clinically relevant in this setting.
Revertant mosaicism is another molecular event related to COL7A1 that is important in the development of treatment strategies. In RDEB, revertant mosaicism, which gives rise to clinically normal patches of skin, is due to the re-expression of C7 resulting from the occurrence of secondary mutations that reverse the primary mutation in COL7A1. Our group was among the first to identify this phenomenon in RDEB.6 The reasons for the reversion are unknown. In the case of EB, it might be that the severe scarring, which involves excessive cell proliferation, could lead to new mutations, which in the case of an already mutated COL7A1 gene in DREB, might exert a beneficial effect by counteracting the effect of the primary mutation. Because of their greater adherence, keratinocytes altered by the revertant mutation might acquire a selective advantage, giving rise to clinically normal patches of skin. These patches can also be used as a source of corrected autologous cells for cell-based therapies, as discussed below.
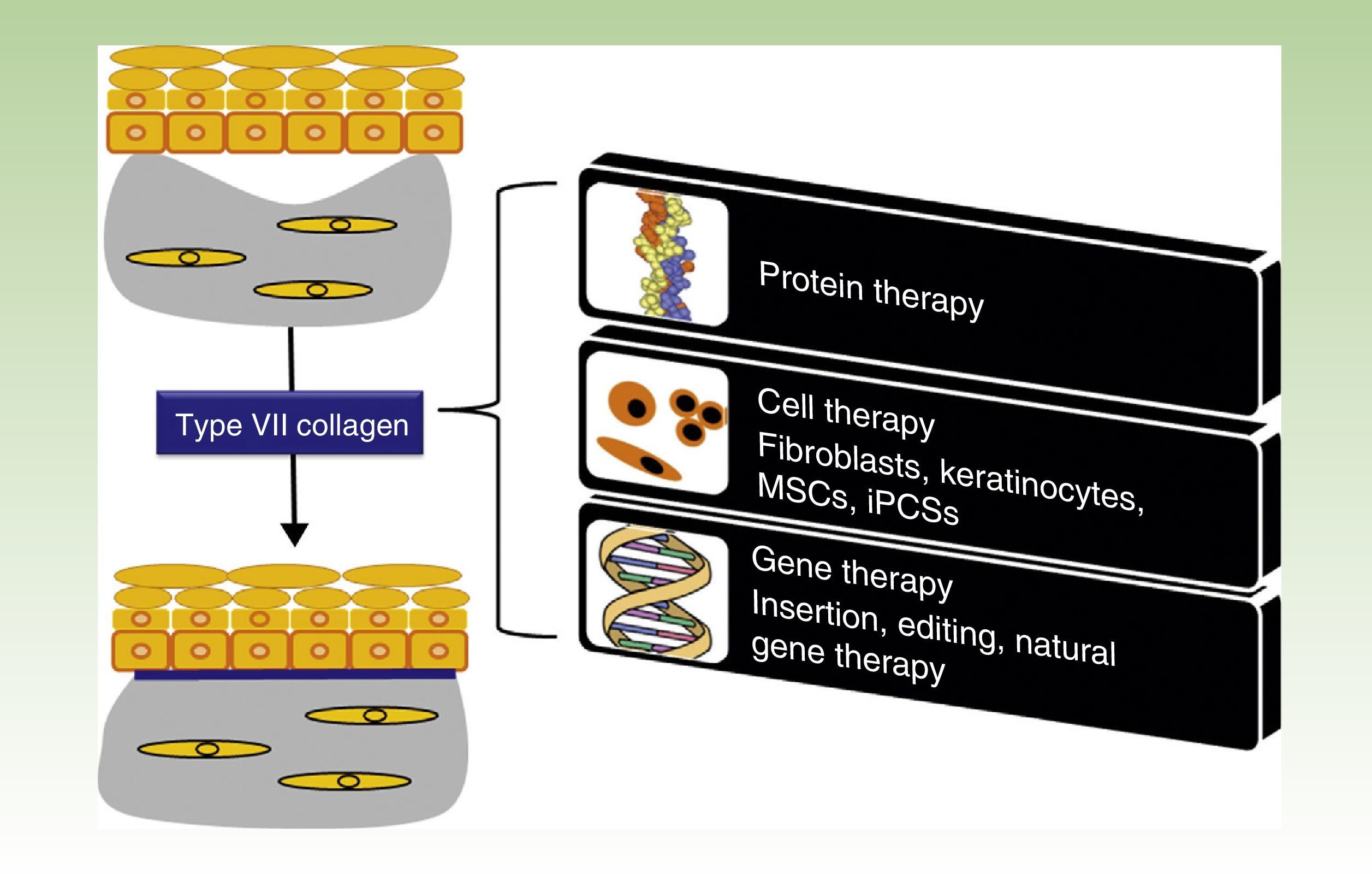
One Disease, Numerous TreatmentsFig. 1 shows the different treatment strategies that are currently being evaluated for RDEB and are described in detail in the following sections.
Treatment strategies for recessive dystrophic epidermolysis bullosa. Protein-, cell- and gene-based therapies (boxes on the right) converge on the production of type VII collagen at the dermal-epidermal junction to correct the bullous phenotype (upper left panel) and restore dermal-epidermal adhesion (lower left panel). MSCs indicate mesenchymal stem cells; iPSCs, induced pluripotent stem cells.
A priori, the most evident approach to correcting C7 and other protein deficiencies would be to administer the absent or reduced protein. In view of the molecular characteristics and solubility of C7 and its localization in tissues, it was initially thought that it would be difficult to administer this protein and furthermore to ensure that it reached its specific expression site. However, preliminary studies by Woodley and Chen and their group demonstrated that the protein monomer was sufficiently soluble to be used in both subcutaneous solutions for local injections and intravenous solutions for systemic treatments. Using RDEB mouse models, they showed that the injected protein was capable of migrating to the basement membrane and correcting the bullous phenotype.7 Systemic administration would have a beneficial effect in that it would lead to improvements not only at the level of the skin, but also at the level of the internal epithelia, which when affected can make it difficult for patients with RDEB to eat solid food. Based on the above work, Lotus Tissue Repair Inc, a company recently acquired by Shire Biopharmaceuticals, is working on the production of recombinant C7, although no formal clinical trials have yet been launched.
Cell TherapyBoth keratinocytes and dermal fibroblasts can secrete C7. In conditions of homeostasis, keratinocytes are the main source of C7 at the dermal-epidermal junction. Nevertheless, C7 cells of mesenchymal origin are currently being used in several cell therapy strategies, with varying degrees of clinical implementation, in the setting of RDEB. One such protocol based on infusion of bone marrow or umbilical cord blood from a healthy HLA-matched/histocompatible donor has been postulated as having significant curative potential. As with systemic administration, this approach also has the advantage of potentially being able to improve internal epithelia in addition to skin. Over 10 patients have been treated with this method to date (ClinicalTrials.gov identifier NCT01033552) and the results appear promising.8,9 The duration of the treatment effect remains to be seen, and further investigation is needed to elucidate the underlying mechanisms, as there is no certainty regarding the cell type that mediates the treatment effect. There is evidence that cells with tropism for affected areas might not be hematopoietic lineage cells (CD34+) but rather mesenchymal precursors positive for platelet-derived growth factor.10 This is promising, as CD34+ cell transplantation is not free of serious adverse effects (conditioning treatment and graft-versus-host disease [GVHD]), and furthermore, these effects can be exacerbated by the already weakened state of health in many of these patients.
Another treatment option is the use of allogeneic fibroblasts from healthy donors. Unlike keratinocytes, these cells may be relatively well tolerated by the recipient's immune system. As already mentioned, fibroblasts produce C7, but in lower quantities than keratinocytes. Bruckner-Tuderman's group in Germany reported that healthy fibroblasts injected intradermally into an RDEB mouse model were capable of producing C7 in vivo, and that this migrated to the basement membrane, where it improved dermal-epidermal adhesion.11,12 These preclinical observations were subsequently confirmed in patients in whom C7 (at levels of 30%-40% of those found in the basement membrane of control individuals) produced by fibroblasts derived from healthy donors was capable of reducing skin fragility when injected locally into patients with RDEB.13,14 The effect seen in these studies, however, was short-lasting, probably due to silent, immune-mediated allogeneic fibroblast rejection.
Mesenchymal stem cell (MSC) therapies also deserve special mention. MSCs are pluripotent cells that can be isolated from bone marrow, subcutaneous fat, umbilical cord blood, and placenta.15 Lipoaspirate cells are the easiest to isolate, cultivate, and expand.16 The capacity of MCSs to migrate to injured zones and stimulate tissue regeneration is now seen as an essential component of their therapeutic potential and explains the enormous interest in evaluating their potential in the treatment of degenerative and inflammatory diseases in clinical research settings. The therapeutic effect of MCSs when administered systemically and locally is currently being investigated in over 200 clinical trials at different stages of development targeting a range of diseases including rheumatoid arthritis, Crohn disease, GVHD, myocardial infarction, multiple sclerosis, and spinal cord damage.17 The finding that the cell population responsible for the therapeutic effect observed following bone marrow transplantation in patients with RDEB might be of mesenchymal origin led to MSC transplantation being proposed as an alternative to conventional bone marrow transplantation in RDEB. Such an approach would, on the one hand, eliminate the risk of GVHD and the need for myeloablative conditioning, and on the other hand, pave the way for allogeneic transplantation without the need for histocompatible donors, as several clinical trials have already shown that the immunosuppressive/immunomodulatory effects of MSCs make them invisible to the recipient's immune system. The intradermal administration of MSCs from healthy donors has been found to stimulate the production of C7 at the dermal-epidermal junction (at least for 4 months), reduce new blister formation, and enhance the re-epithelization of persistent wounds in 2 patients with RDEB.18 Nineteen clinical trials investigating RDEB are currently registered in the National Institutes of Health database (www.clinicaltrials.gov). Two of these trials are studying the safety and efficacy of allogeneic MCSs from healthy donors in the treatment of RDEB and are currently in the recruitment phase. One of the trials is being conducted in Japan and is studying the intradermal administration of bone marrow–derived MSCs (UMIN000006723). The other one, underway in the United Kingdom, is evaluating the systemic infusion of allogeneic MSCs, also from bone marrow (ISRCTN46615946). In Spain, our group, in collaboration with researchers from Hospital La Paz in Madrid, is currently in the process of applying for the necessary authorization for a clinical trial based on the infusion of lipoaspirate MSCs. Local cell therapy with MSCs is also an attractive option for treating ulcers in RDEB, as these cells have proven capable of controlling inflammation seen in certain persistent ulcers that poses a major obstacle to skin regeneration. MSCs has also been reported to have a variety of pleiotropic effects (e.g., cytoprotective, antifibrotic, and antimicrobial) that, together with the production of C7, would exert a synergistic effect, favoring more efficient healing.19 Finally, the duration of the therapeutic effect could be substantial considering the long half-life of C7 in vivo.
Also worthy of mention in this section on cell therapy are several trials based on bioengineered tissue products designed for direct application on wounds. So far, however, the results published for various types of organotypic 3D products containing fibroblasts or fibroblasts and keratinocytes have been modest.20,21 A technique by our group involving the combined use of bioengineered skin grafts containing histocompatible (HLA-matched) fibroblasts and keratinocytes have yielded good results in a small number of patients with RDEB (del Río et al., unpublished results). Other promising techniques such as those combining MSCs with fibrin matrices and other biomaterials have yet to be evaluated clinically.
Gene TherapyAs EB and other genetic skin diseases are primarily monogenic disorders, gene therapy is a promising curative strategy. Numerous preclinical studies of ex vivo gene therapy have reported successful results for various forms of EB in the past 12 years, including studies by our group involving mouse models with regenerated skin from patients with RDEB and other forms of EB.22,23 The only clinical study to report successful results to date is that by Mavilio et al.24 in a patient with junctional EB in Italy. In that study, keratinocytes from the patient were transduced with laminin 332 β3 cDNA using a conventional retroviral vector. Simple epidermal grafts containing the genetically modified keratinocytes were then transplanted onto lesions measuring just a few square centimeters on the patient's legs, resulting in restoration of dermal-epidermal adhesion in the treated areas. Additionally, thanks to the long duration of the graft (over 8 years from the time of transplantation), it was possible to demonstrate transfer of the curative gene to the epidermal stem cell population. Several laboratories, including ours, have demonstrated in different preclinical studies the feasibility of ex vivo correction of skin cells (keratinocytes and fibroblasts) from patients with RDEB using integrative viral vectors (retroviruses and lentiviruses) and nonviral vectors including wild type COL7A1 complementary DNA.22,25,26 Two gene therapy protocols for RDEB are currently being investigated in clinical trials in the United States (ClinicalTrials.gov identifier NCT01263379) and Europe (www.genegraft.eu). Using a protocol similar to that described by Mavilio et al.24 in the patient with junctional EB, the US trial is evaluating the safety and efficacy of a conventional retroviral vector25 and a genetically engineered epithelial graft cultured from the patient's skin. The European study, which is funded by the European Union and in which our group is involved, is using a self-activating retroviral vector26 and ex vivo gene therapy combined with skin tissue engineering. The idea is to transplant bioengineered skin containing genetically modified keratinocytes and fibroblasts. There are also other techniques based on nonviral transposon/transposase-mediated gene transfer methods27 or corrections of mutations through RNA repair,28,29 but these are still in the experimental phases. Several aminoglycoside antibiotics are potentially capable of overcoming premature stop codons in mRNA transcripts caused by certain COL7A1 mutations. Accordingly, topical application of these antibiotics could be used in vivo to temporarily correct these mutations and at the same time induce C7 production.30
Because of the adverse effects observed in clinical gene therapy protocols for X-linked severe combined immunodeficiency, in which the gene transfer retroviral vector used activated an oncogene that led to leukemia in some patients,31 gene therapy research in recent years has primarily sought to find methods that offer greater biosafety. The focus was on protocols that would minimize the risk of mutagenesis due to the insertion of a vector carrying the corrective gene, and until relatively recently, the primary goal was to improve the safety of these vectors.23,31 However, the emergence of molecular tools that enable genome editing,32 and thereby minimize genotoxic risks, has revolutionized the field of gene therapy over the last 4 years. These molecular tools are based on nucleases, which are enzymes that cleave DNA into shorter fragments. They do not act randomly, but rather target specific genome sequences through the action of other proteins or RNA that guide the nucleases to the target cleavage site. DNA cleavage triggers repair processes that, for example, through homologous recombination, allow the defective gene to be replaced by an appropriately supplied healthy gene. A report published in 2013 described how a technique based on the above methods was successfully used to correct a COL7A1 mutation.33 The molecular tools used in this case were transcription activator-like effector nucleases introduced into fibroblasts from a patient with RDEB.33 Another tool currently in use is CRISPR (clustered regularly interspaced short palindromic repeats), which consists of an RNA recognition sequence that acts as a guide for a nuclease (in this case Cas9) that cuts the DNA at the position marked by the guide RNA. The CRISPR/Cas9 system has been used in the correction of mutations through homologous recombination34 and in the genetic modification of stem cells.35,36 As mentioned previously, the COL7A1 c.6527insC mutation causes a premature stop codon that prevents mRNA from encoding C7. There are also other techniques apart from homologous recombination for correcting the reading frame of mRNA in COL7A1. One of these, which is currently being tested in our laboratory, targets mRNA processing machinery with the aim of excluding exon 80, which is where the mutation lies. The ultimate aim is to restore the reading frame (lost due to the insertion of an extra C in exon 80) and ensure that the mRNA transcription of COL7A1 is completed. The result would be restoration of the production of C7, which would remain functional even though it lacks the 12 amino acids encoded by exon 80 (Larcher, unpublished results). Reading frame correction by targeted genome editing has been successfully used with cells from patients with Duchenne muscular dystrophy.37
Finally, recent advances in skin tissue engineering and experimental surgical procedures have made it possible to analyze the in vivo potential of individual modified epidermal stem cells genetically selected for their proliferative capacity in culture. In this area, our group has achieved long-term human skin regeneration using genetically modified epidermal stem cell clones known as holoclones.38 Similar approaches will be necessary to test the regenerative capacity of cells carrying the COL7A1 locus previously corrected by gene editing.
Natural Gene TherapyThe previously described gene reversion phenomenon can be considered a natural, spontaneous form of gene therapy involving no manipulation. Revertant cells can be harvested from a biopsy of reverted skin and used to produce cultured autologous skin grafts, similarly to those used in severe burn cases. The idea, however, is not to produce large sheets of skin, but rather enough skin to treat wounds that do not heal and have a propensity to progress to carcinoma, as occurs in severe, generalized RDEB. This approach has been successfully employed in a preclinical study in which bioengineered skin grafts containing revertant keratinocytes from a patient with junctional EB were introduced into immunodeficient mice. The results showed that even though the grafts contained just 20% of revertant cells, the regenerated human skin in the mice was clinically normal.39 In a recent study, Jonkman's group demonstrated the feasibility of this technique in a patient with junctional EB, although they did not use tissue-engineered skin.40 Instead, they simply transplanted punch biopsy specimens from revertant skin patches onto persistent wounds. Not only did the transplanted skin last, but there was also clinical improvement, with no additional blisters developing in the treated areas.
Future TreatmentsWhile gene therapy is advancing thanks to the use of innovative strategies such as the correction of mutations via genome editing, the field of tissue regeneration is undergoing a major revolution with the advent of induced pluripotent stem cells (iPSCs). These cells have almost identical characteristics to embryonic stem cells, but can be generated using adult cells from skin or other tissues via the expression of Yamanaka factors.41 Under suitable conditions, which in some cases have yet to be determined, iPSCs can differentiate into any cell type desired and in almost unlimited quantities.41 These advances have obvious implications for the field of dermatology, as one of the possibilities offered by iPSCs is the production of autologous skin when skin cells (and in particular epidermal stem cells) are in short supply, as occurs in EB and other diseases.42 Several groups have reported the successful generation of iPSCs from cells derived from patients with RDEB before and after genetic correction.33,43 A recent study demonstrated that by combining state-of-the-art technologies, it was possible to correct RDEB fibroblasts through homologous recombination using transcription activator-like effector nucleases and then reprogram these into iPSCs, which then differentiate into keratinocytes.33 iPSCs were also recently obtained from naturally corrected revertant RDEB cells, which would enable the production of autologous epithelial and mesenchymal cells for cutaneous and/or systemic treatments.44
Final ConsiderationsRare diseases have been receiving unprecedented recognition and publicity in social, political, and health care spheres in recent years thanks largely to the efforts of patient associations and other nongovernmental organizations. This heightened awareness has led to an increase in funding for research into rare diseases. Within its new Horizon 2020 programme, for example, the European Union has significantly stepped up funding for research in this area. In Spain, new research programs for the period 2013-2016 are following in a similar direction, albeit with local restrictions. The work being done within CIBERER (Centre for Biomedical Research on Rare Diseases) (www.cibere.es), an initiative launched by Instituto de Salud Carlos III that encompasses a network of important centers investigating rare diseases, has made a key contribution to the advances being made in this field. Much of the progress in RDEB and other genetic skin diseases, which is somewhat greater than in other rare diseases, is largely due to this renewed impetus. These advances are likely to bear fruits in the years to come, but to ensure that this happens, it is essential to keep attracting public and private investment in rare diseases.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
FundingThe authors are grateful for funding received from the Spanish Ministry of Economy and Competitiveness through projects SAF2013/43475-R (MDR) and ISCIII PI14/00931 (FL) and from the Community of Madrid through projects S2010/BMD-2420 (MDR) and S2010/BMD-2359 (FL).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank all the members of Termeg (www.uc3m.termeg.es) and the Epithelial Biomedicine Division at CIEMAT for their contribution to the work cited in this review.
Please cite this article as: Larcher F, Del Río M. Estrategias terapéuticas innovadoras para la epidermólisis bullosa distrófica recesiva. Actas Dermosifiliogr. 2015;106:376–382.