Dermatopathology includes a long list of disorders, some of which have very similar histopathology. Immunohistochemistry is an important auxiliary tool for diagnosis and differential diagnosis, and for predicting the outcome of many skin tumors. It is also the main technique for determining the origin of a tissue or the differentiation of neoplastic cells. In many cases, immunohistochemistry provides a more accurate diagnosis of the different processes that infiltrate the skin. This review examines the role of immunohistochemistry in studying the differentiation and biological behavior of the majority of tumors that can involve the skin. We review the immunoperoxidase techniques, discuss the utility of the most commonly used antibodies, and highlight a number of diagnostic problems in which immunohistochemistry may be very useful. In each case, the goal is to reach a specific and definitive diagnosis. In the first part of this review, we examine the antibodies that determine the different cell-differentiation profiles of skin tumors.
La dermatopatología incluye una larga lista de entidades, algunas con una histopatología muy similar. La immunohistoquímica representa una importante herramienta de ayuda en el diagnóstico, diagnóstico diferencial y pronóstico de muchas de las neoplasias cutáneas. La inmunohistoquímica es también la mejor técnica para determinar el origen de un tejido o la diferenciación de las células neoplásicas. En muchos casos la inmunohistoquímica permite un diagnóstico más preciso de los distintos procesos infiltrando la piel. Este artículo revisa el papel de la inmunohistoquímica en el estudio de la diferenciación y el comportamiento biológico de la mayoría de las neoplasias que pueden afectar a la piel. Se revisan las técnicas de inmunoperoxidasa, se discute la utilidad de los anticuerpos utilizados con mayor frecuencia y se presentan una serie de problemas diagnósticos en los que la immunohistoquímica puede resultar muy útil. En cada caso, la finalidad es llegar a un diagnóstico concreto y definitivo. En la primera parte de esta revisión se estudian los anticuerpos que exploran las distintas líneas de diferenciación de las neoplasias cutáneas.
Immunohistochemistry has become an essential diagnostic tool in dermatopathology. The term covers a group of immunostaining techniques in which labeled antibodies are used to detect the presence of antigens in cells or tissues. The principle of immunohistochemistry lies in the ability of antibodies to bind specifically to their respective antigens. The resulting reaction can only be visualized when the antibody is labeled with a substance that absorbs or emits light or produces color.
Immunofluorescence techniques rely on the use of fluorescein-labeled markers, which, when exposed to ultraviolet light, emit visible light of varying wavelengths depending on the nature of the compound used. Direct immunofluorescence is used widely in the diagnosis of skin diseases, a field in which it has very specific indications. In particular it is used to diagnose bullous diseases, vasculitis, and certain types of tumors. Although it is more sensitive than immunostaining, immunofluorescence has certain disadvantages, including loss of fluorescence over time, the need for a specialized light microscope, and poor visualization of morphologic features. Furthermore, the resulting reactions need to be photographed each time for documentation purposes.
Immunoperoxidase techniques involve the use of enzyme labels that convert a colorless substrate into a colored one. The most widely used enzymes are peroxidase and alkaline phosphatase, and the most widely used substrates are diaminobenzidine, amino ethylcarbazole, and nitroblue tetrazolium, which, respectively, produce a brown, red, and blue color. These markers can be attached, or conjugated, directly to the primary antibody, or indirectly using secondary antibodies or substances such as biotin and protein A. The range of commercially available antibodies is growing daily and it is now possible to find markers for a broad spectrum of antigens.
Immunohistochemistry has become an increasingly important histopathologic tool over the past 20 years and is now a key part of routine practice. In particular, it is becoming increasingly important for the diagnosis and classification of a growing list of tumors.
Below is a summary of the main applications of immunohistochemistry techniques today:
- 1
Determination of the origin or degree of differentiation of a tumor
- 2
Refinement of prognosis
- 3
Differentiation between benign and malignant tumors
- 4
Determination of the molecular architecture of a tissue
- 5
Detection of infectious agents in cells or tissues
Table 1 shows the most common immunohistochemical markers used in dermatopathology, classified by the type of differentiation they are used to detect.
Main Immunohistochemical Markers Used in Dermatopathology.
| Epithelial differentiation |
| Cytokeratins |
| EMA |
| CEA |
| GCDFP antigen |
| Mesenchymal differentiation |
| Pan-mesenchymal: vimentin |
| Muscle |
| Actin |
| Muscle-specific |
| Sarcomeric |
| α-smooth muscle |
| Caldesmon |
| Calponin |
| Desmin |
| Myogenin |
| Myosin |
| Endothelial |
| CD31 |
| CD34 |
| WT-1 |
| Podoplanin |
| LYVE-1 |
| PROX-1 |
| VEGFR-3 |
| GLUT-1 |
| FLI-1 |
| ERG |
| Neuroectodermal differentiation |
| S-100 protein |
| HMB-45 |
| Melan-A |
| Neuron-specific enolase |
| Neurofilaments |
| Hematopoietic differentiation |
| Differentiation markers |
| -Cytokeratins (AE1/AE3) |
| -CD45 |
| Lineage markers |
| B-cell |
| CD20 |
| CD79a |
| T-cell |
| CD45RO |
| CD3 |
| Natural killer cell: CD56 |
| Markers of clonality |
| κ or γ light-chain restriction |
| Monotypic proliferation |
| CD4 |
| CD8 |
| Antigen loss: CD5, CD7 |
| Antigen coexpression: CD4+CD8, CD20+CD5, CD20+CD43 |
| Translocation markers |
| Bcl-2 |
| ALK |
| Cyclin D1 |
| Activation markers |
| CD30 |
| EMA |
| Proliferation markers |
| Ki-67 |
| Origin markers |
| Bcl-6 |
| CD10 |
| Accessory cell markers |
| CD1a |
| CD6 |
Abbreviations: ALK, anaplastic lymphoma kinase; Bcl, B-cell lymphoma; CEA, carcinoembryonic antigen; EMA, epithelial membrane antigen; ERG, v-ets erythroblastosis virus E26 oncogene homolog (avian); FLI-1, friend leukemia virus integration 1; GCDFP, gross cystic disease fluid protein; GLUT-1, glucose transporter 1; HMB-45, human black melanoma 45; VEGFR-3, vascular endothelial growth factor receptor 3; WT-1, Wilms tumor 1.
Cytokeratins are the largest group of intermediate filaments. They are filamentous proteins which, together with other filaments, form the eukaryotic cytoskeleton. They have numerous functions, including maintenance of epithelial structure, protection from injury, and communication with other cytoplasmic components. They are expressed in pairs, with expression patterns varying according to location, and classified numerically from 1 to 20 according to their molecular weight and isoelectric point.
There are 2 major groups of cytokeratins: simple epithelial cytokeratins (CK7, CK8, CK18, CK19, and CK20) and more complex epithelial cytokeratins, such as those found in the skin (CK5/6, CK10, CK14, and CK15). A second classification system distinguishes between acidic (or type I) cytokeratins, which generally correspond to low-molecular-weight cytokeratins (CK9-CK20) and basic (type II) cytokeratins (CK1-CK8), which generally have a high molecular weight. Monoclonal antibodies, with different levels of specificity, have been developed for these different cytokeratins1 (Table 2).
Cytokeratin Specificity.
| Epithelial type | Type II | Type I | Distribution |
| Simple epithelia | 8 | 18 | Secretory and parenchymatous cells |
| 7 | 19 | Ductal and gastrointestinal epithelia | |
| 20 | Gastrointestinal epithelia, Merkel cells of the skin, taste buds of the oral mucosa | ||
| Stratified squamous epithelia | 5 | 14 | Basal cells of squamous and glandular epithelia, myoepithelia, mesothelium |
| 15 | Squamous epithelia | ||
| 8 | 18/19 | Noncornifying stratified squamous epithelia | |
| Suprabasal cells | 1 | 10/11 | Epidermis (suprabasal compartment) |
| 9 | Epidermis of palms and soles | ||
| 2e | Epidermis (high layers) | ||
| 2p | Gingiva and hard palate | ||
| 3 | 12 | Corneal epithelium | |
| 4 | 13 | Nonkeratinizing stratified squamous epithelia of internal organs | |
| 6 | 16,17 | Cytokeratin 17 is typically expressed in basal cells of complex epithelia |
Source: Adapted from Chu et al..14
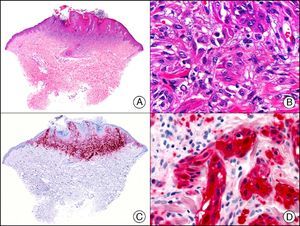
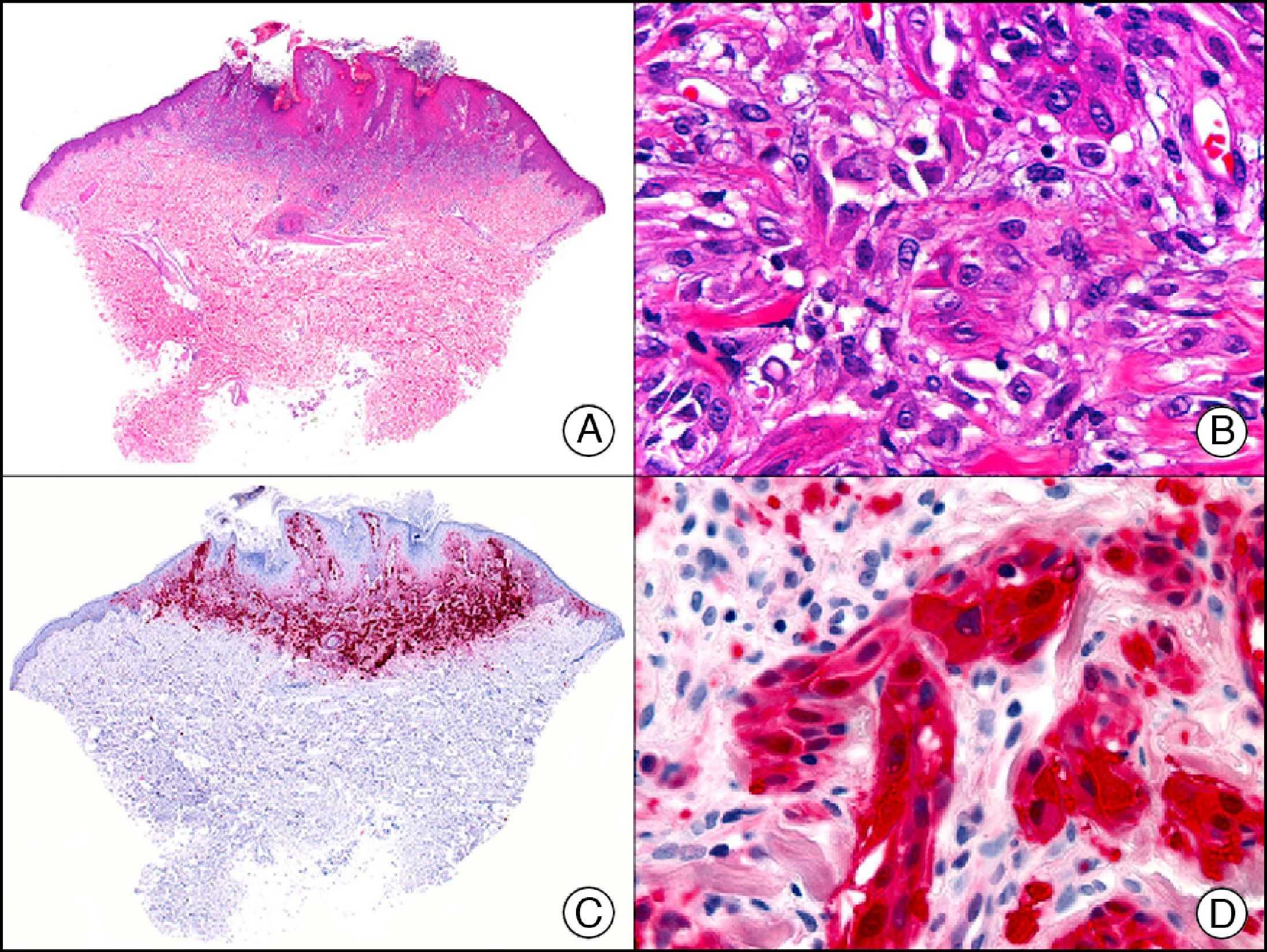
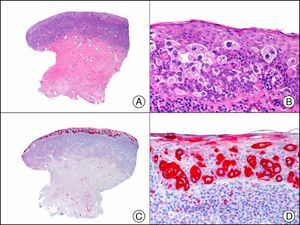
The pan cytokeratin AE1/AE3 is a cocktail of antibodies that recognizes a wide range of cytokeratins of different molecular weights. It is very useful for identifying epithelial tumors, classifying these by degree of differentiation, and detecting micrometastases. In the skin, AE1/AE3 labels the epidermis, the eccrine glands, and the folliculosebaceous-apocrine unit. It has high diagnostic sensitivity as it is capable of identifying most carcinomas (Fig. 1), even poorly differentiated ones. It also recognizes several epithelial mesenchymal tumors formed by epithelioid cells (e.g., mesothelioma, synovial sarcoma, and epithelioid sarcoma), as these all have cells containing large quantities of intermediate filaments in their cytoplasm. Cytokeratin AE1 recognizes acidic cytokeratins (CK10, CK15, CK16, CK19). It is a characteristic simple epithelial cytokeratin and in the skin the cytokines it recognizes are expressed only in the basal layer of the epidermis.1,2 Cytokeratin AE3, in turn, recognizes basic cytokeratins (CK1-CK8) and is characteristic of transitional epithelial cells and squamous cell carcinomas. The cytokines are expressed throughout the epidermis.1,2
Squamous cell carcinoma. A, Low-power magnification (×10). B, Detail of neoplastic cells, showing vesicular nuclei with prominent nucleoli and abundant eosinophilic cytoplasm (×400). C, The same sample studied immunohistochemically with the pan cytokeratin antibody AE1/AE3. Note the positive staining of the neoplastic cells, the epithelium of the epidermis, and the dermal appendages (×10). D, High-power magnification of neoplastic cells stained with AE1/AE3 (×400).
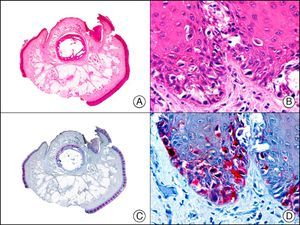
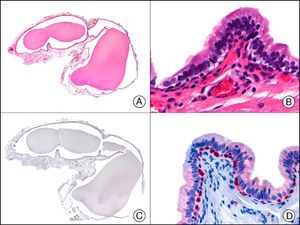
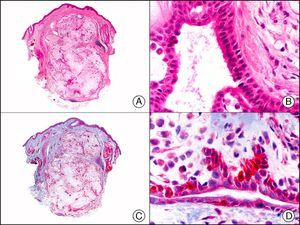
CAM 5.2 recognizes low-molecular-weight cytokeratins in glandular tumors.3 There are conflicting data in the literature regarding the cytokeratins recognized by CAM 5.2. In early reports, CAM 5.2 was considered to react with CK8, CK18, and CK19.4 It is now known, however, that it reacts with CK8 and, less strongly but more specifically, with CK7 and also that it does not react with CK18.5–7 In healthy skin, CAM 5.2 labels the eccrine and apocrine units, but not all the layers of normal epidermis. It can be used to demonstrate the epithelial origin of highly undifferentiated tumors and is therefore a useful histological tool for distinguishing between poorly differentiated carcinomas, lymphomas, melanomas, and sarcomas. It is also a useful marker of metastatic skin tumors with neuroendocrine differentiation, such as carcinoid tumors or small-cell lung cancer, and of primary cutaneous neuroendocrine tumors, such as Merkel cell carcinoma, and their metastases. Finally, it is able to detect low-molecular-weight glandular epithelium in the epidermal epithelium in mammary Paget disease (MPD) and extramammary Paget disease (EMPD) (Fig. 2). It is a very useful histopathologic tool for differentiating between pigmented MPD and pagetoid malignant melanoma.7,8
Extramammary Paget disease. A, Low-power magnification (×10). B, Detail showing numerous isolated large and atypical cells, with abundant basophilic cytoplasm, dotted throughout the epidermis (×400). C, The same sample studied immunohistochemically with CAM 5.2 (×10). D, Note the positive staining of neoplastic cells and negative staining of epidermal keratinocytes (×400).
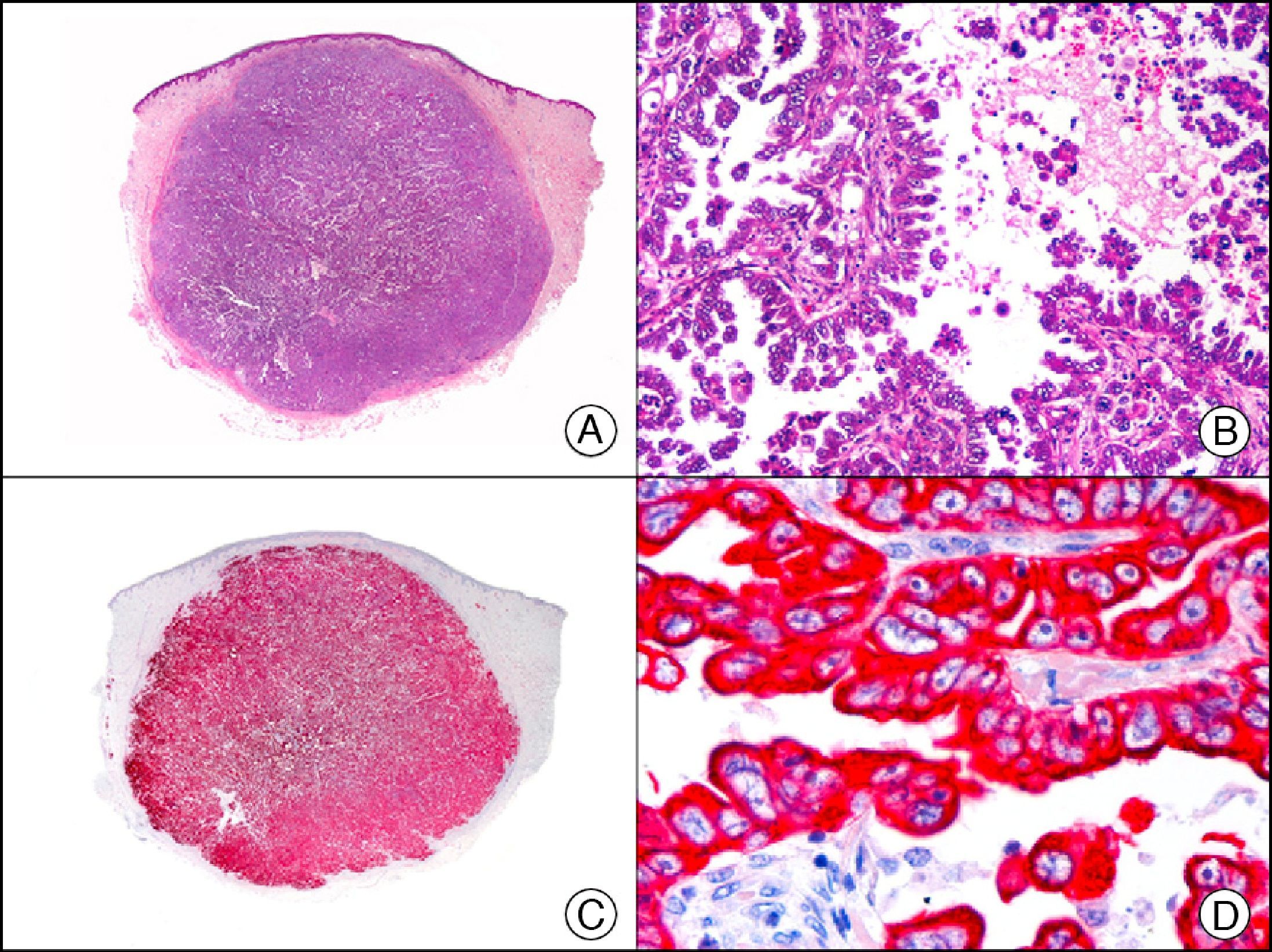
The anti-cytokeratin antibody 5bH11 recognizes the low-molecular-weight cytokeratins CK8 and CK18. CK8 is a basic cytokeratin that, together with the acidic CK18, is probably the most abundant intermediate filament protein. It is expressed in all simple epithelial cells, including those of the thyroid gland, the breast, the gastrointestinal tract, the respiratory tract, and the urogenital tract. In oncology, it is useful for detecting adenocarcinomas and carcinomas derived from nonkeratinizing squamous epithelium (Fig. 3). CK8/18 expression is now used in the molecular subclassification of breast cancers, with both prognostic and predictive significance, along with other markers such as estrogen receptors, human epidermal growth factor receptor 2, CK5/6, E-cadherin, c-Kit, and epidermal growth factor receptor. CK8/18 positivity, for example, is observed in ductal but not lobular breast cancer.
Cutaneous metastasis of papillary carcinoma of the endometrium. A, Low-power magnification (×10). B, Detail showing papillae lined with atypical epithelial cells (×200). C, The same sample studied immunohistochemically with cytokeratin (CK) 8/18 (×10). D, Detail of CK8/18-positive neoplastic cells (×400).
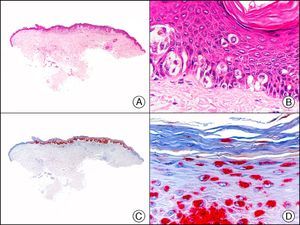
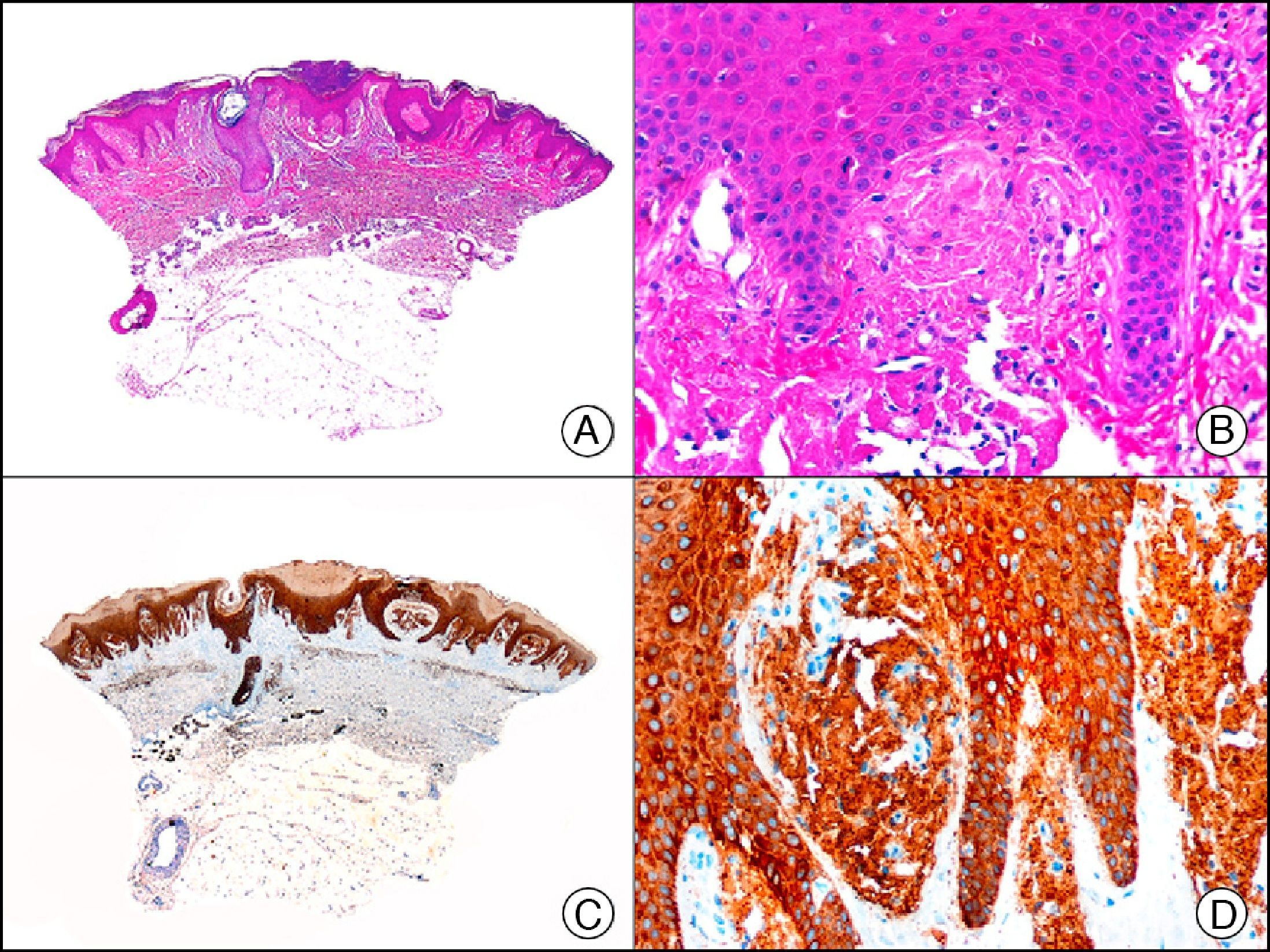
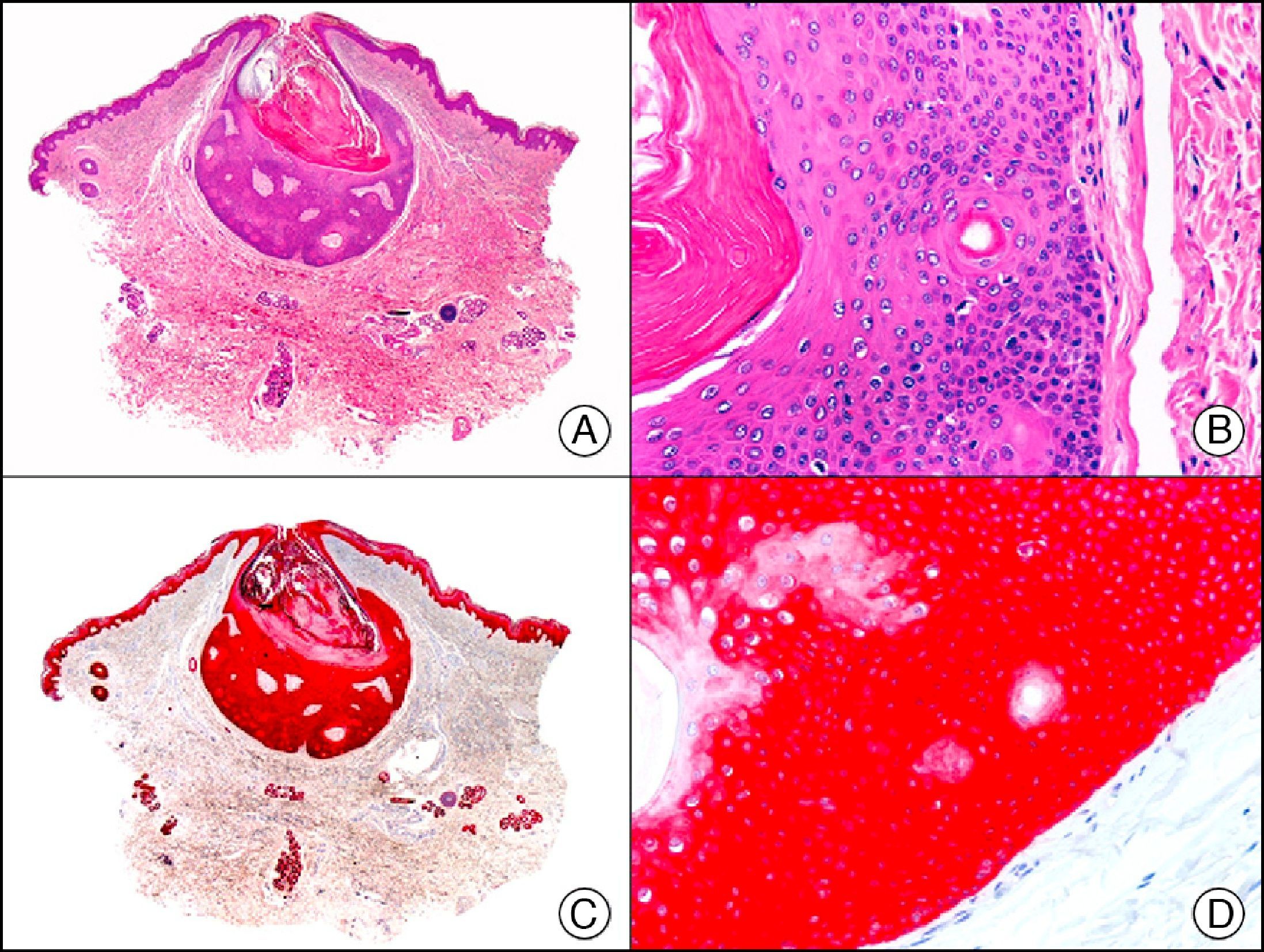
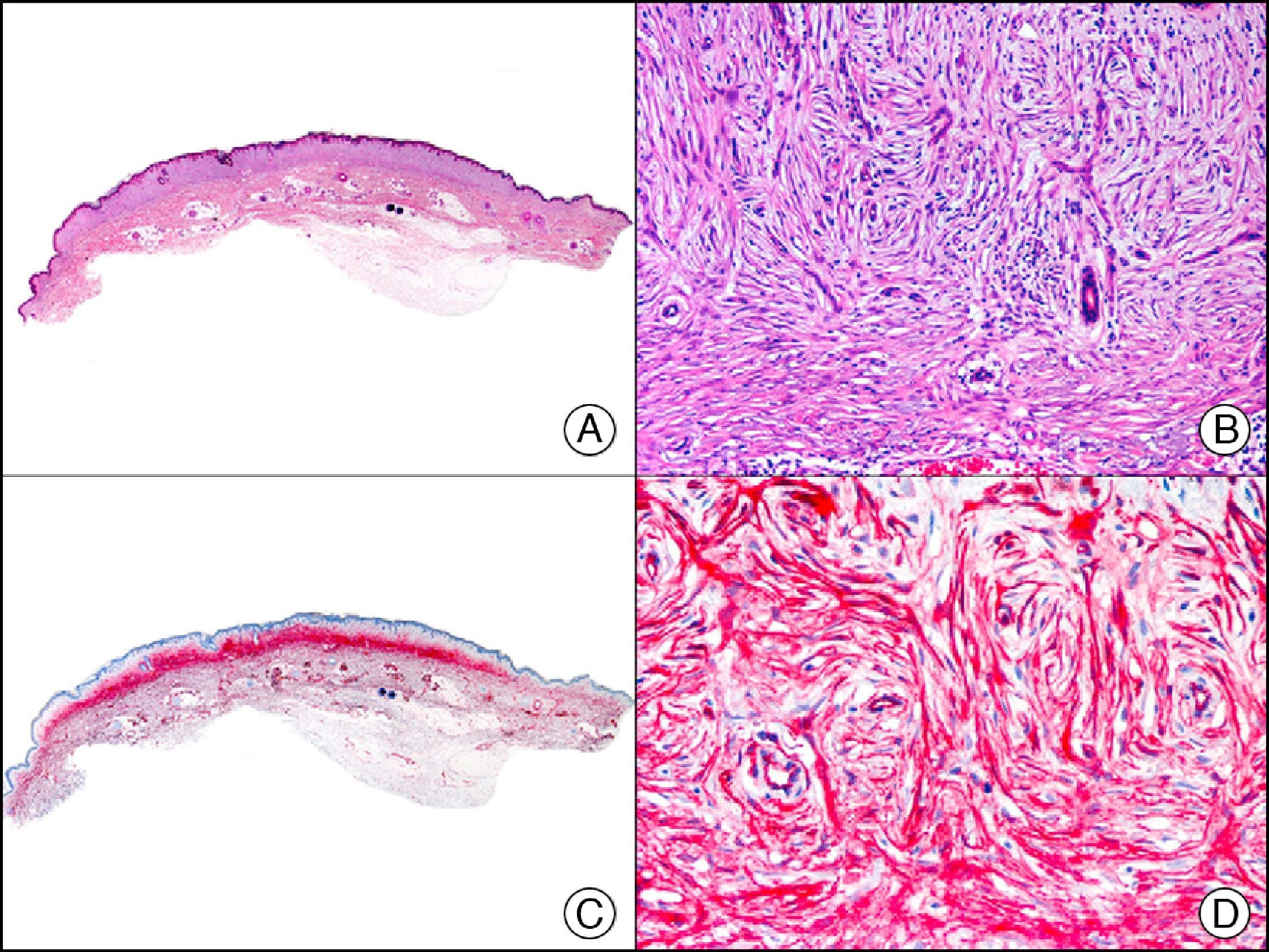
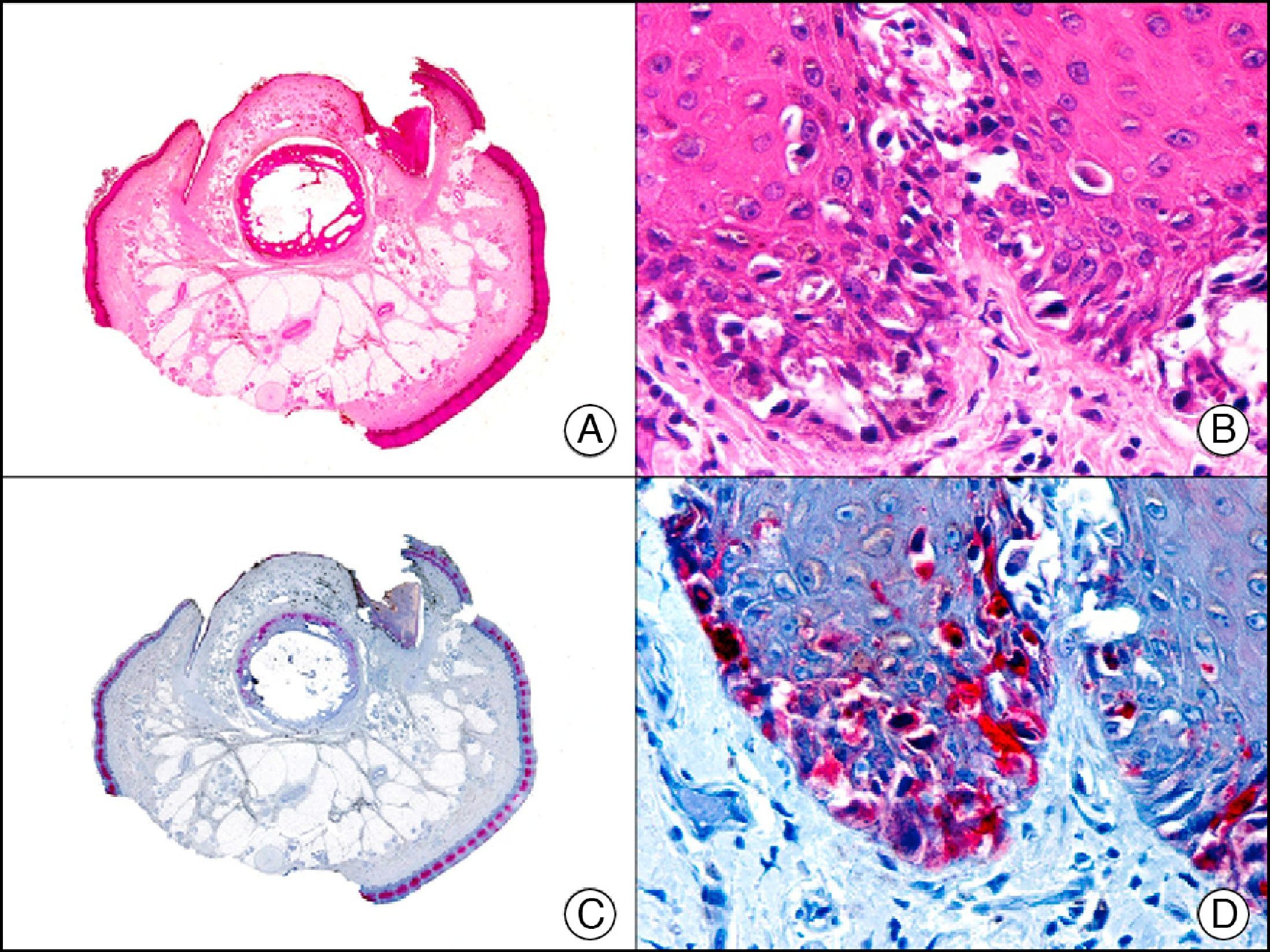
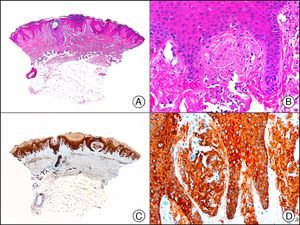
34βE12/CK903 is the most widely used antibody to identify high-molecular-weight cytokeratins. It recognizes CK1, CK10, and CK14, which are expressed in ductal and squamous epithelia, basal cells, and myoepithelial cells.3 This antibody labels adenocarcinomas of the breast, the pancreas, the biliary tree, and the salivary glands, and also squamous cell and transitional cell carcinomas. In dermatopathology, CK903 is very useful for demonstrating the epithelial origin of amyloid deposits in the dermal papillae in cases of macular and lichen amyloidosis (Fig. 4). In cutaneous tumors, it is positive in squamous cell carcinoma and adnexal tumors. CK903 was also recently proposed as a diagnostic tool for distinguishing between atypical squamous cell carcinoma and atypical fibroxanthoma.9
Lichen amyloidosis. A, Low-power magnification (×10). B, Detail of epidermal hyperplasia and deposits of amyloid substance in the papillary dermis (×200). C, The same sample stained with cytokeratin (CK) 903. Note the positive staining of both the epidermis and the amyloid substance in the papillary dermis, demonstrating its epithelial origin (×10). D, Detail of CK903-positive amyloid substance in the papillary dermis (×200).
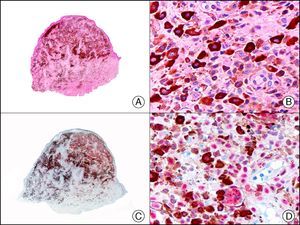
MNF-116, another pan cytokeratin antibody, recognizes CK5, CK6, CK8, CK17, and CK19. It labels a wide range of epithelial tissues, from simple glandular epithelia to stratified squamous epithelia. In normal skin, staining is particularly intense in the basal layer of the epidermis and skin appendages.3 MNF-116 is therefore positive in benign and malignant epithelial tumors and negative in all mesenchymal and melanocytic tumors. It is very useful in the identification of certain rare histopathologic variants of undifferentiated squamous cell carcinoma, such as spindle cell carcinoma and sarcomatoid carcinoma10,11 (Fig. 5).
Highly undifferentiated squamous cell carcinoma. A, Low-power magnification (×10). B, Detail of neoplastic cells, showing spindle-cell morphology and no evidence of keratinization (×400). C, The same sample studied immunohistochemically with the pan cytokeratin MNF-116. Note the positive reaction in the epidermis and the epithelium of the skin appendages (positive internal control) (×10). D, High-power magnification (×400). Note the positive staining in the cytoplasm of several neoplastic cells in addition to the eccrine ducts.
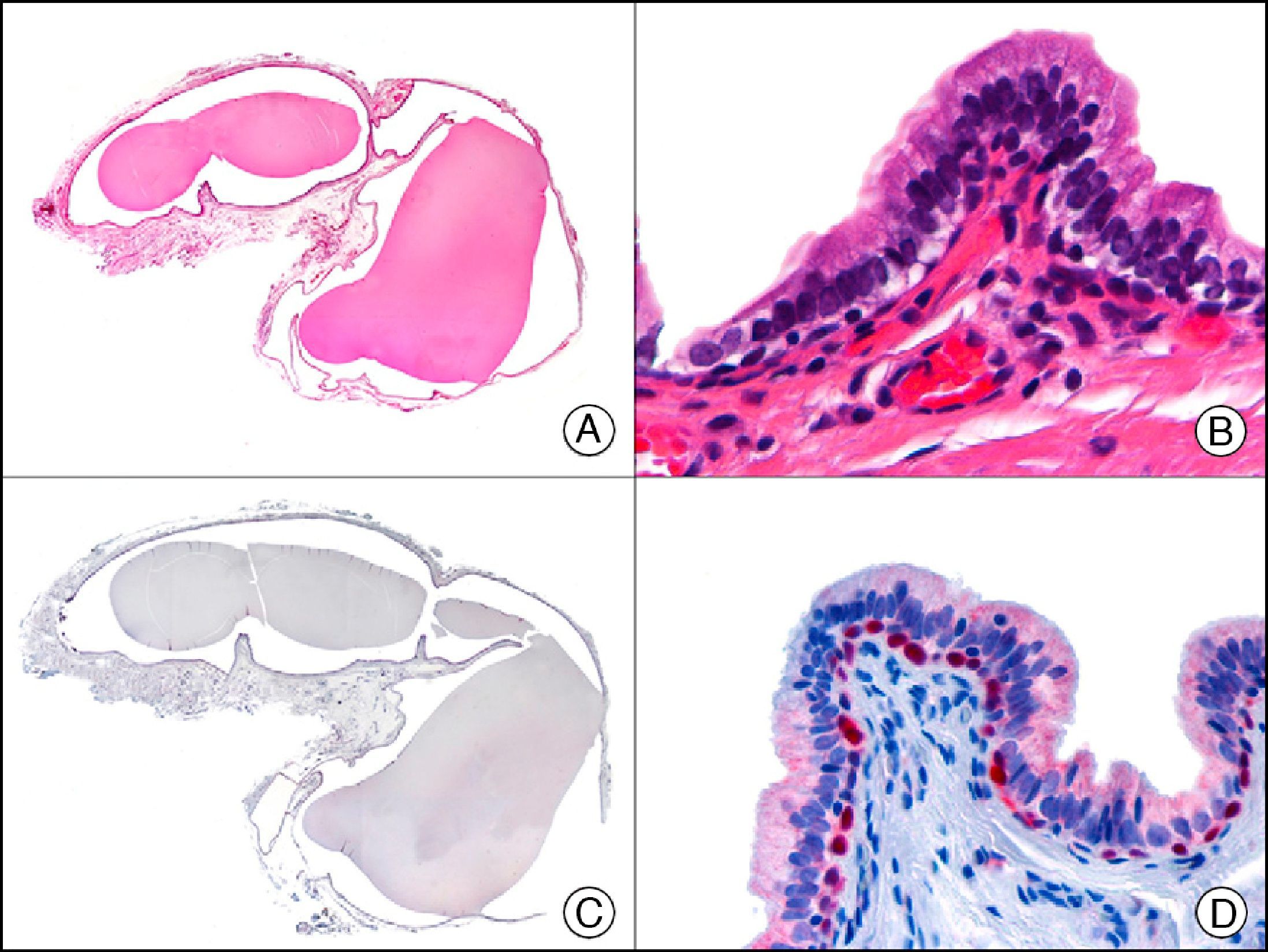
CK7 is expressed in a wide variety of simple epithelial tissues. It is normally negative in cutaneous squamous cell carcinomas and positive in nonkeratinizing squamous cell carcinomas, such as that of the cervix. It is a highly sensitive marker and has good specificity for the identification of neoplastic cells in MPD and EMPD3,12,13 (Fig. 6).
Epidermotropic metastasis from carcinoma of the bladder in the mucosa of the glans, mimicking extramammary Paget disease. A, Low-power magnification (×10). B, High-power magnification showing large cells with pleomorphic nuclei and abundant cytoplasm scattered through the epithelium (×400). C, The same sample studied immunohistochemically with cytokeratin (CK) 7 (×10). D, High-power magnification showing CK7 positivity in intraepithelial neoplastic cells (×400).
CK20 is expressed in fewer tissues than CK7. It is positive in transitional cell carcinomas, Merkel cell carcinoma (with a highly characteristic paranuclear globular pattern of staining) (Fig. 7), and adenocarcinomas of the bile system, the pancreas, the colon, and the rectum. It is therefore extremely valuable in the immunohistochemical study of primary and metastatic skin tumors. Its usefulness in distinguishing primary (or true) EMPD from secondary EMPD (epidermotropic metastasis that mimics EMPD) is the subject of considerable debate as it seems to be widely positive in both conditions, although to a lesser extent than CK7.13 Together with CK7, it is used in antibody panels designed to determine the primary site of the tumor in patients with skin metastasis of unknown origin.10
Merkel cell carcinoma. A, Low-power magnification (×10). B, High-power magnification of cytologic features of neoplastic cells, showing round, basophilic nuclei, with granular chromatin, absent nucleoli, and scant cytoplasm (×400). C, The same sample studied immunohistochemically with cytokeratin (CK) 20 (×10). D, High-power magnification of CK20–positive neoplastic cells in a paranuclear globular pattern (×400).
CK5/6 recognizes high-molecular-weight cytokeratins. It is a useful marker of squamous differentiation and is positive in the epidermis and epithelium of skin appendages and in multiple benign and malignant skin adnexal tumors (Fig. 8). It also appears to produce weak staining in up to 30% of metastasis of adenocarcinoma and is therefore of some value in differentiating between primary cutaneous tumors and cutaneous metastases from internal malignancies.3,14 The majority of mesotheliomas, squamous cell carcinomas, basal cell carcinomas, and transitional cell carcinomas are reactive with this antibody. Adenocarcinomas, by contrast, are largely nonreactive.
Poroma. A, Low-power magnification (x10). B, Detail showing that most of the neoplastic cells are poroid but also that there are some cuticular cells visible around a small duct (×200). C, The same sample studied immunohistochemically with cytokeratin (CK) 5/6 (×10). D, High-power magnification showing CK5/65 staining of neoplastic cells (×200).
CK14 is positive in epithelial tumors, such as squamous cell carcinoma and basal cell carcinoma (Fig. 9), as well as in adnexal neoplasms, such as poromas, sebaceous adenomas, proliferating trichilemmal tumors, and trichoblastomas. It is negative in adnexal adenocarcinomas.
CK8 is negative in squamous cell and basal cell carcinoma and in the myoepithelium, and positive in certain tumors with eccrine and apocrine differentiation, such as syringoma and apocrine hidradenoma (Fig. 10).
Apocrine (clear-cell) hidradenoma. A, Low-power magnification (×10). B, High-power magnification of a cluster of neoplastic cells with clear-cell morphology (×400). C, The same sample studied immunohistochemically with cytokeratin (CK) 8 (×10). D, Detail of CK8–positive neoplastic cells (×400).
p63, which is also called KET or p73L, is a protein member of the p53 family of tumor suppressor genes. It can act as a transcription factor, regulating cell cycle progression, maintaining proliferative capacity, or inducing apoptosis, depending on the environmental stimulus received by the cell. It is found mainly in the basal layer of the stratified squamous epithelium and the transitional epithelium. In the skin, it is strongly expressed in the nucleus of basal and suprabasal epidermal cells and in the nucleus of myoepithelial cells surrounding the secretory coils of eccrine and apocrine glands. p63 is not expressed in dermal fibroblasts, smooth muscle fibers, Schwann cells, or endothelial cells. It is, however, expressed in basal cells of the epithelial layers of the cervix, vagina, urothelium, bronchial mucosa, and prostate glands and in myoepithelial cells of the seromucous glands (Fig. 11). p63 expression has been observed in several types of squamous carcinomas, such as those of the skin, the lungs, and the cervix, as well as in papillary thyroid carcinoma. In poorly differentiated spindle cell carcinoma, which is normally negative for many cytokeratins, p63 is a useful diagnostic marker and of particular value for differentiating this entity from atypical fibroxanthoma, spindle cell melanoma, and cutaneous leiomyosarcoma.3 It is expressed in most skin adnexal carcinomas and their metastases but not in visceral adenocarcinomas or their skin metastases; its discriminative value is similar to that of podoplanin (D2-40).15
Cyst on the foreskin of a newborn. A, Low-power magnification (×10). B, Detail of epithelium of cyst wall, showing a peripheral layer of cuboidal cells and a luminal layer of columnar cells (×400). C, The same sample studied immunohistochemically with p63 (×10). D, High-power magnification showing positive reaction in the nuclei of the peripheral cells, which are probably myoepithelial cells (×400).
EMA (epithelial membrane antigen) is a glycosylated protein expressed on the surface of various glandular epithelial cells and in tumors derived from these cells. In normal skin, it is expressed in sebaceous glands (in the excretory duct and sebocytes). Histologically, the antibody produces staining of the cytoplasmic lipidic microvacuoles, explaining why EMA is one of the most widely used immunohistochemical markers to investigate sebaceous differentiation in cutaneous tumors (Fig. 12). In most cases, both the secretory portion and the excretory duct of eccrine and apocrine glands stain for EMA, but there have been reports of negative results for the eccrine duct.16–19 EMA is also expressed in perineural cells. It is not expressed in normal squamous epithelium, but is frequently positive in squamous cell carcinoma.19,20 It is positive in plasma cells and recognizes neoplastic cells in EMPD, allowing these to be clearly distinguished from neighboring epidermal keratinocytes.2 Finally, EMA has been observed in neoplastic cells of tumors with diverse lineages of differentiation, such as meningiomas, mesotheliomas, multiple types of mesenchymal tumors, and even certain lymphomas, such as CD30+ anaplastic large-cell T-cell lymphoma.
Sebaceous carcinoma. A, Low-power magnification (x10). B, High-power magnification showing findings indicative of sebaceous differentiation in the form of immature sebocytes and sebaceous ducts (×400). C, The same sample studied immunohistochemically with EMA (epithelial membrane antigen. Note the positive staining of the normal sebaceous glands of the dermis (positive internal control)(×10). D, High-power magnification of EMA-positive areas with more evident sebaceous differentiation (×400).
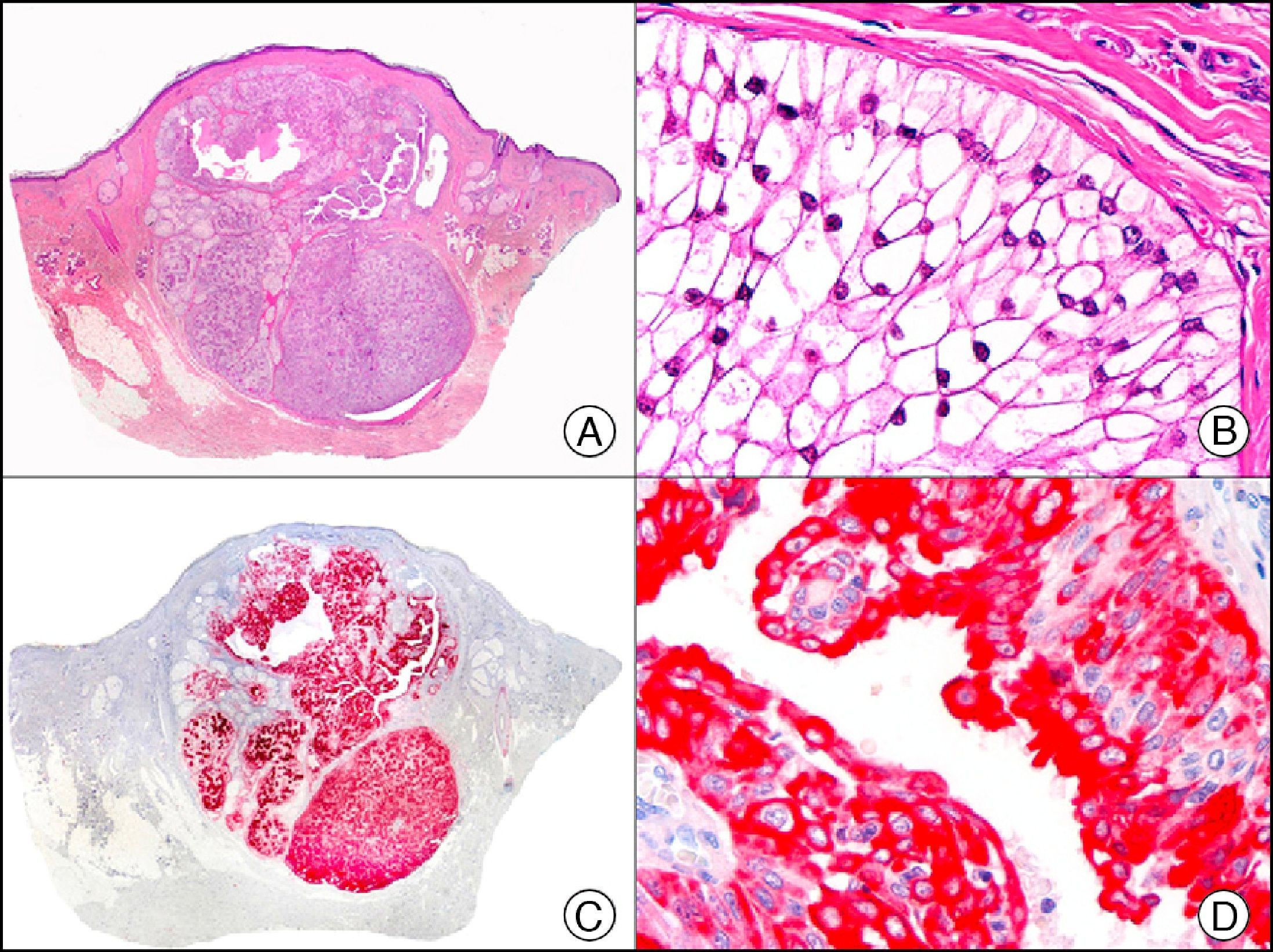
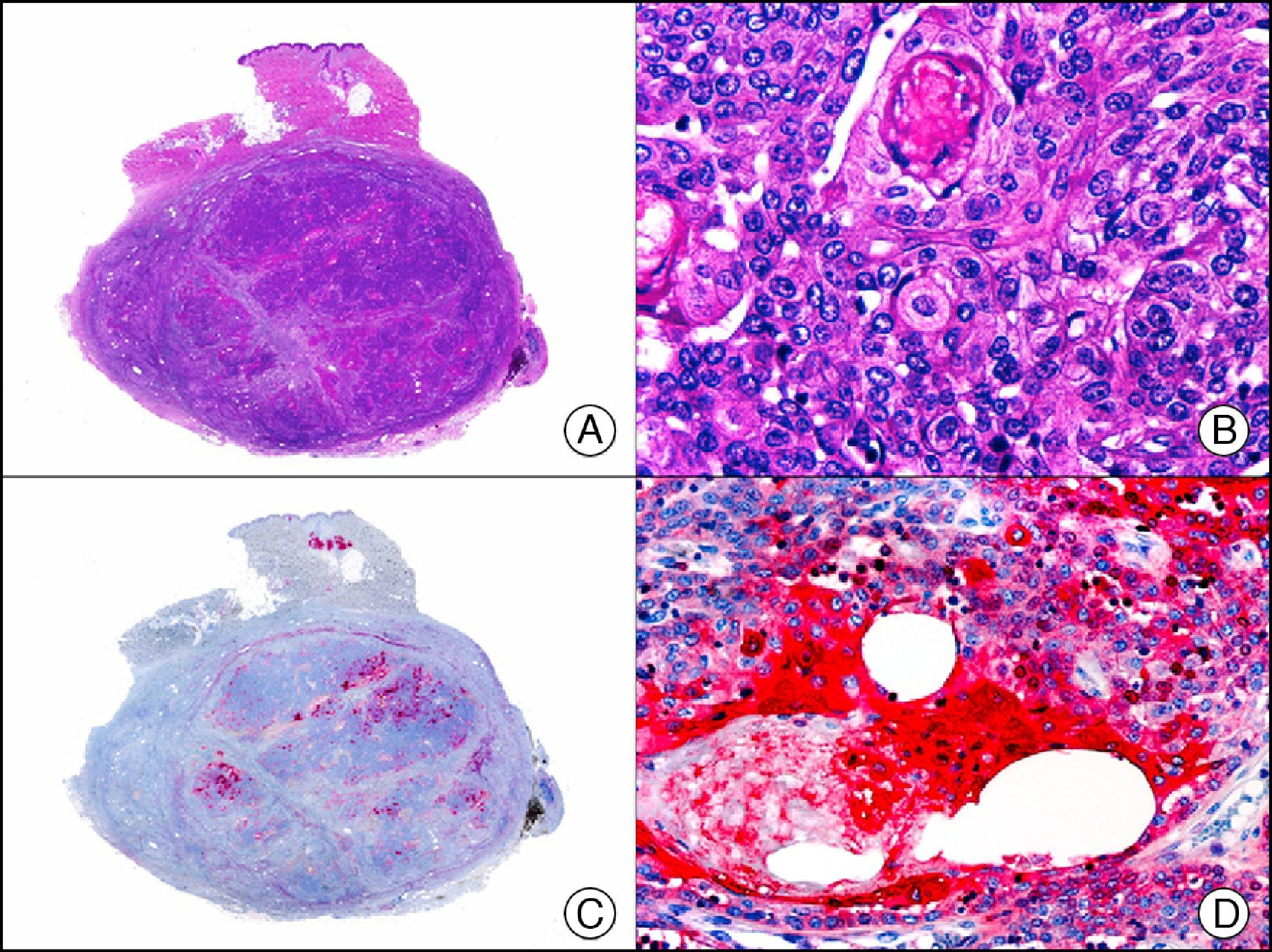
CEA (carcinoembryonic antigen) is another glycosylated protein that in normal skin is expressed in the eccrine and apocrine glands, but not in the sebaceous glands.2 In the case of eccrine glands, it is expressed in the secretory and excretory portions of the gland. In the case of apocrine glands, however, strong positivity is observed at the luminal edge of the excretory duct, but CEA is often absent from the secretory portion21 CEA is an important marker of primary cutaneous adenocarcinomas (Fig. 13) and cutaneous metastases from internal adenocarcinomas; it is also expressed in neoplastic cells of MPD and EMPD.8
GCDFPs (gross cystic disease fluid proteins) are a family of proteins first identified in the fluid of breast cysts in patients with fibrocystic breast disease. The luminal edges of the secretory tubules and the excretory ducts of the eccrine and apocrine glands show intense immunoreactivity for GCDFP.2 Variable expression patterns have also been observed in many ductal and secretory tumors with eccrine and apocrine differentiation (Fig. 14) and in neoplastic cells in MPD and EMPD.12,22
Primary histiocytoid cell carcinoma of the eyelid. A, Low-power magnification (×10). B, Detail of histiocytoid aspect of neoplastic cells, with several cells showing cytoplasmic vacuolization (×400). C, The same sample studied immunohistochemically with GCDFP-15 (gross cystic disease fluid protein 15) (×10). D, Detail of GCDFP-15-positive neoplastic cells (×400).
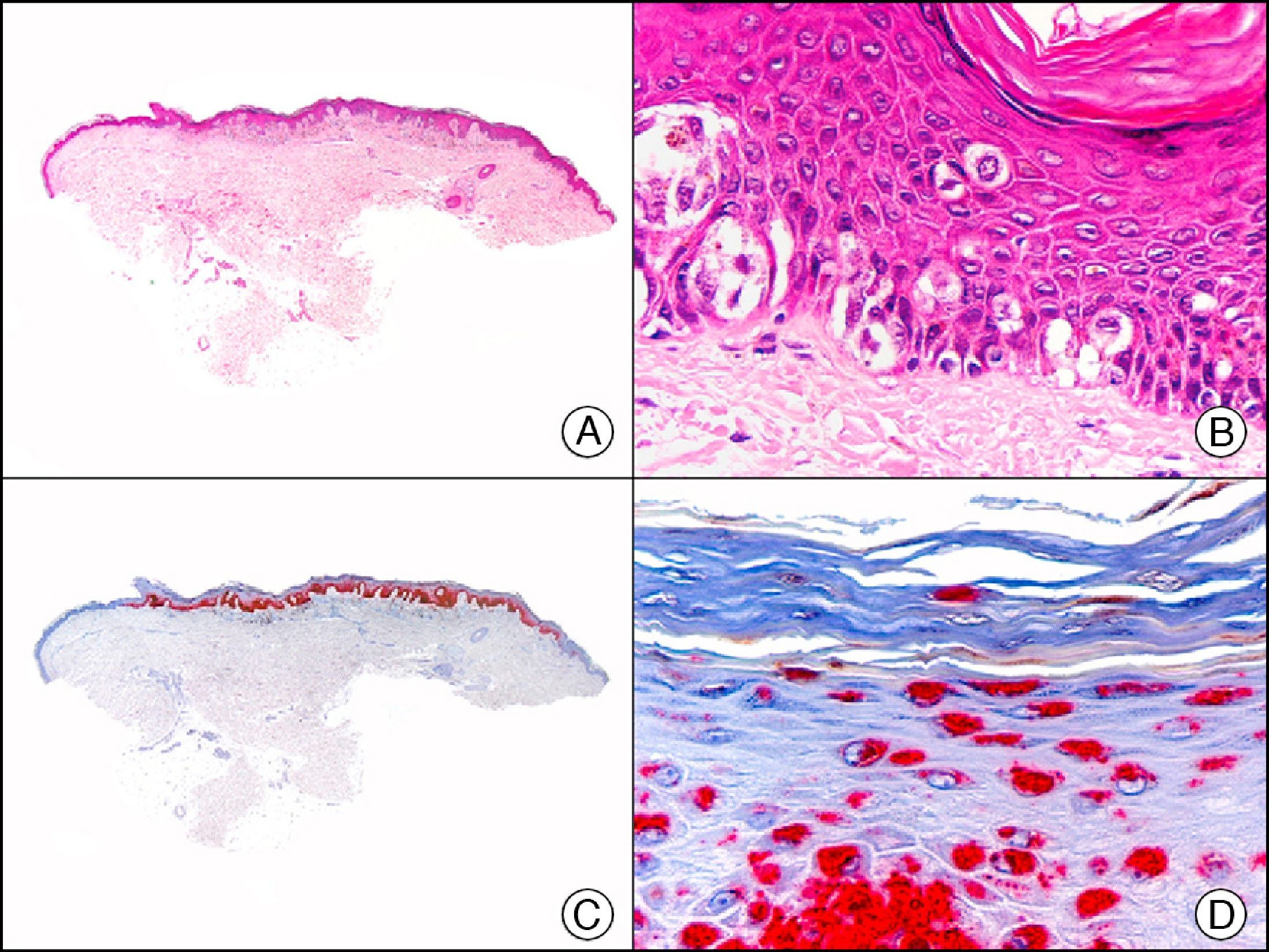
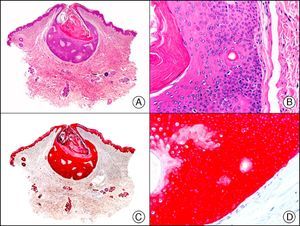
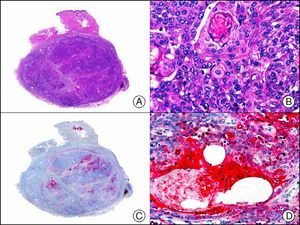
Calretinin is a 32-kDa calcium-binding protein that belongs to the EF-hand family of proteins, which are characterized by a sequence of amino acids that fold into a helix-loop-helix structure. Calretinin has 6 EF-hand domains and 5 calcium-binding sites. Its main function is to act as a buffer and prevent excessive accumulation of intracellular calcium. It has been observed in different phases of the cell cycle in a wide variety of normal and neoplastic tissues. In normal skin, it is expressed in the innermost layer of the outer root sheath of the hair follicle (the companion layer), in the sebaceous duct, and in the secretory portion of eccrine glands; it is not expressed in the secretory portion of apocrine glands or in eccrine or apocrine excretory ducts.23–25 In skin tumors, calretinin is positive in proliferations with differentiation towards the outer root sheath of the hair follicle (trichilemmal differentiation), sebaceous ductal differentiation, and eccrine secretory differentiation (e.g., eccrine mixed tumors).23–25 It is noteworthy that calretinin is strongly positive in neoplastic cells in very varied skin tumors, such as granulosa cell tumors, keratoacanthoma (Fig. 15), and squamous cell carcinoma.
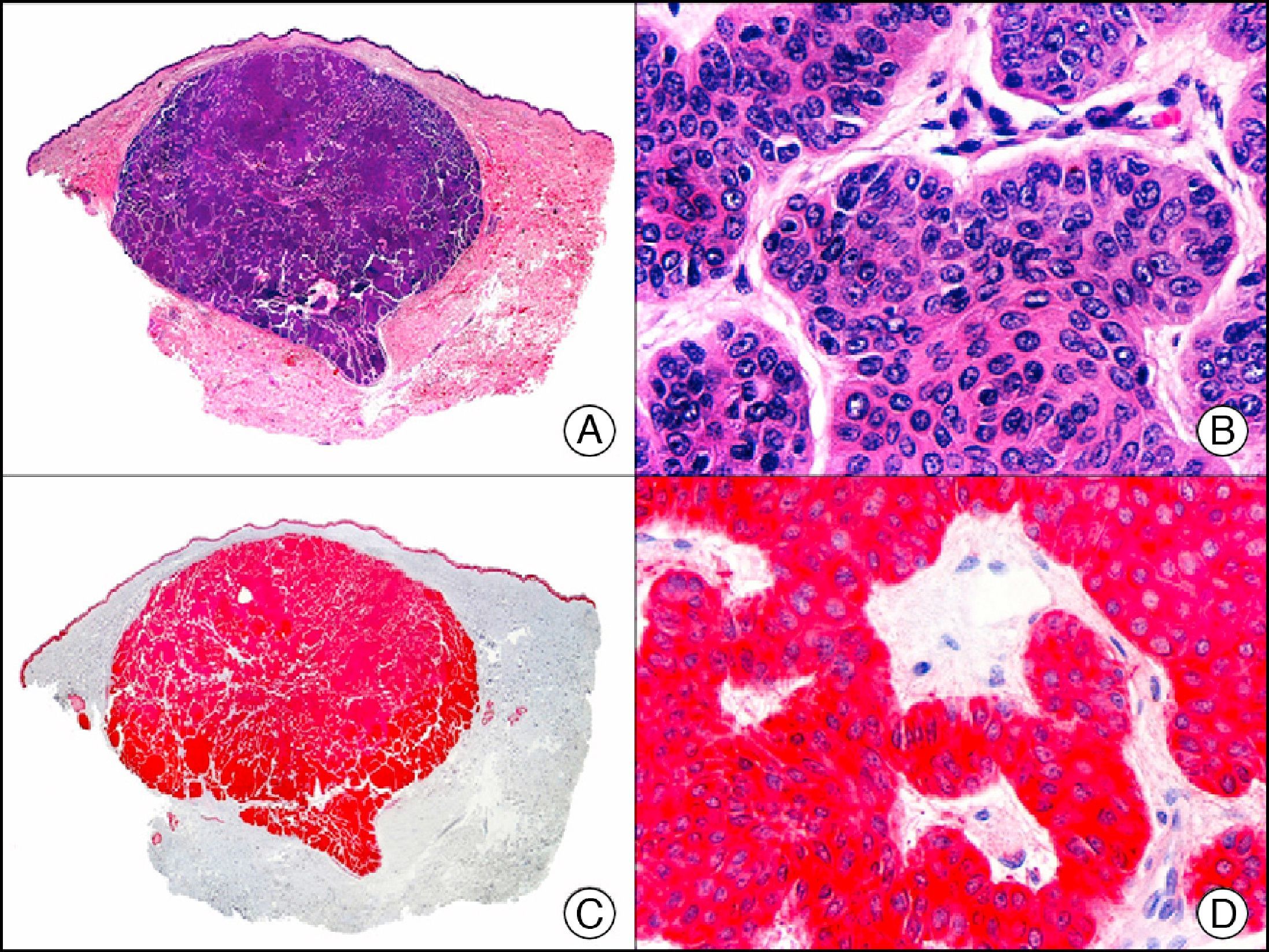
HCG (human chorionic gonadotropin) is a 40-kDa glycoprotein composed of an α and a β subunit. The β subunit (β-hCG) is secreted by the placenta and is normally only detectable in blood during pregnancy. It the most important marker of gestational trophoblastic cells, and is expressed in syncytiotrophoblasts and intermediate trophoblasts, but not in cytotrophoblasts. In choriocarcinoma, this marker stains syncytiotrophoblasts intensely and diffusely (Fig. 16). The detection of β-hCG in nontrophoblastic tumors may indicate an aggressive course.26
Cutaneous metastasis of testicular choriocarcinoma. A, Low-power magnification (×10). B, Detail of syncytiotrophoblastic cells (×400). C, The same sample studied immunohistochemically with β-HCG (β-human chorionic gonadotropin) (×40). D, Detail of β-HCG-positive syncytiotrophoblastic cells (×400).
Vimentin is present in cultures of practically all embryonic cells and most adult cells, whatever their lineage. During embryogenesis, it is progressively replaced by cell-specific intermediate filaments, but is maintained in mesenchymal cells. In normal skin, this marker is expressed in melanocytes at the dermal-epidermal junction, as well as in fibroblasts, dendrocytes, blood and lymph vessels, smooth muscle in the dermis, and adipocytes in the hypodermis. In oncology, it occurs in all types of sarcomas, melanomas, and spindle cell carcinomas and in some other types of carcinoma.1 It is also positive in most mesotheliomas and gliomas. In dermatopathology, vimentin is useful for demonstrating mesenchymal differentiation and for supporting a diagnosis of melanocytic differentiation in poorly differentiated amelanotic melanomas.2 The diagnostic value of this ubiquitous immunostain, however, is increasingly being called into question due to its poor specificity. Because of its high sensitivity, however, it has been proposed by several authors as a useful tool for determining the adequacy of tissue for immunohistochemical study with other markers, as vimentin positivity indicates good preservation of antigens in tissue.
Muscle Differentiation MarkersSmooth muscle actin, as an individual marker, is the most useful type of actin for histological diagnosis. It is expressed in smooth muscle, myofibroblasts, pericytes, glomus cells, and myoepithelial cells and therefore has high sensitivity for detecting differentiation towards smooth muscle and myofibroblasts in cutaneous tumors1,2 (Fig. 17). Like other markers, however, it also occurs in many nonmuscle tumors.
Cutaneous glomangioma. A, Low-power magnification (x10). B, High-power magnification of neoplastic cells around the vascular lumen, with round monomorphous nuclei and scant cytoplasm (×400). C, The same sample studied immunohistochemically with α-SMA (smooth muscle actin) (×10). D, Detail of α-SMA-positive neoplastic cells (×400).
Desmin is an intermediate filament found in skeletal, cardiac, and smooth muscle cells, as well as in submesothelial fibroblasts, endometrial stromal cells, and some lymph node dendritic cells. It is a specific marker of muscle differentiation, particularly differentiation towards skeletal or striated muscle, and recognizes both benign tumors (leiomyomas [Fig. 18] and rhabdomyomas) and malignant tumors (leiomyosarcomas and rhabdomyosarcomas). Staining intensity varies from one muscle tissue to another, and, for instance, is much stronger in the uterine muscle than in the erector pili muscle. Weak focal staining is observed in tumors with myofibroblastic differentiation, such as fibromatosis, dermatofibrosarcoma protuberans, and certain types of malignant fibrous histiocytoma. It is therefore very useful for differentiating smooth muscle tumors from tumors of myofibroblastic origin, as desmin tends to be very weak or even absent in the latter.1
Cutaneous angioleiomyoma. A, Low-power magnification (×10). B, High-power magnification of neoplastic cells showing elongated nuclei with blunt ends and eosinophilic cytoplasm (×400). C, The same sample studied immunohistochemically with desmin (×10). D, Detail of desmin-positive neoplastic cells (×400).
Caldesmon, a cytoskeletal protein found in muscle cells, is responsible for inhibiting calcium-dependent contractions in smooth muscle. It is present in many types of cells in a low-molecular-weight form. Its high-molecular-weight form is believed to be expressed exclusively in visceral and vascular smooth muscle and myoepithelial cells. Staining of myoepithelial cells is variable, and in a series of tumors supposedly with myoepithelial differentiation studied by Watanable et al.27 all the neoplastic myoepithelial cells were negative. Caldesmon would appear to be a more specific smooth muscle marker than muscle-specific actin, desmin, or α-smooth muscle actin. Its main value in dermatopathology is its ability to discriminate between true smooth muscle tumors (Fig. 19) and myofibroblastic tumors.28 Caldesmon also produces intense staining of glomus cells in glomus tumors and glomulovenous malformations.
Cutaneous angioleiomyoma. A, Low-power magnification (×10). B, High-power magnification of neoplastic cells showing elongated nuclei with blunt ends and eosinophilic cytoplasm (×400). C, The same sample studied immunohistochemically with caldesmon (×10). D, Detail of caldesmon-positive neoplastic cells (×400).
Calponin is a smooth muscle–specific protein, similar to calmodulin, that binds strongly to actin in a calcium-independent manner and appears to be involved in the regulation of smooth muscle contraction. It is used as a marker of differentiation towards parenchymal and vascular smooth muscle but has also been observed in myofibroblasts in the desmoplastic stroma, angiomatoid malignant fibrous histiocytoma, myoepithelial cells of sweat gland tumors (Fig. 20) and salivary gland tumors, and myoepithelial carcinoma of soft tissue. Immunoreactivity has also been reported in proliferating cells of tumors of widely diverse origins, such as neurothekeoma, glomus tumors, myofibrosarcoma of the bone, and even synovial sarcoma (in which it shows focal staining).29,30
Apocrine mixed tumor. A, Low-power magnification (×10). B, Detail of elongated ductal structures lined with a double layer of cells (×400). C, The same sample studied immunohistochemically with calponin (×10). D, Detail of peripheral layer of calponin-positive cells lining the ducts, supporting the myoepithelial origin of these cells (×400).
Myogenin is a human protein that plays an important role in regulating muscle differentiation. It is a member of a family of proteins known as myogenic regulatory factors (MRFs); these are transcription factors with basic helix-loop-helix domains that act sequentially in the myogenic differentiation process. MyoD, Myf5, myogenin, and MRF4 (Myf6) are all important members of the MRF family. Myogenin is expressed exclusively in skeletal muscle cells, and expression levels appear to be inversely proportional to the degree of cell differentiation. It has also been observed in some cases of rhabdomyosarcoma (Fig. 21), fibromatosis, infantile myofibromatosis, synovial sarcoma, and leiomyosarcoma.31
Myosin is a fibrous protein that interacts with actin in the regulation of muscle contraction. It is the most abundant protein in skeletal muscle, accounting for between 60% and 70% of all muscle proteins, and is the major component of thick filaments. It is therefore a useful marker of differentiation towards smooth and striate muscle.
Endothelial Differentiation MarkersCD31 is a transmembrane glycoprotein that is expressed in discontinuous endothelial cells of lymph vessels, macrophages, platelets, and all continuous endothelial cells (of arteries, arterioles, venules, veins, and nonsinusoidal capillaries). It is involved in the binding of endothelial cells with each other and with lymphocytes, and is the most sensitive and specific marker of endothelial differentiation. It recognizes neoplastic cells in the vast majority of benign vascular tumors (Fig. 22) and in over 90% of hemangioendotheliomas and angiosarcomas. It is also very useful in the evaluation of tumor angiogenesis. However, its expression in the macrophages of the stroma of vascular and nonvascular tumors can cause confusion and give rise to diagnostic errors.32
Infantile hemangioma in subcutaneous tissue. A, Low-power magnification (×10). B, High-power magnification of capillary vessels lined with monomorphous endothelial cells, without atypia (×200). C, The same sample studied immunohistochemically with CD31 (×10). D, Detail of CD31-positive endothelial cells lining the lumen of the capillary vessels (×200).
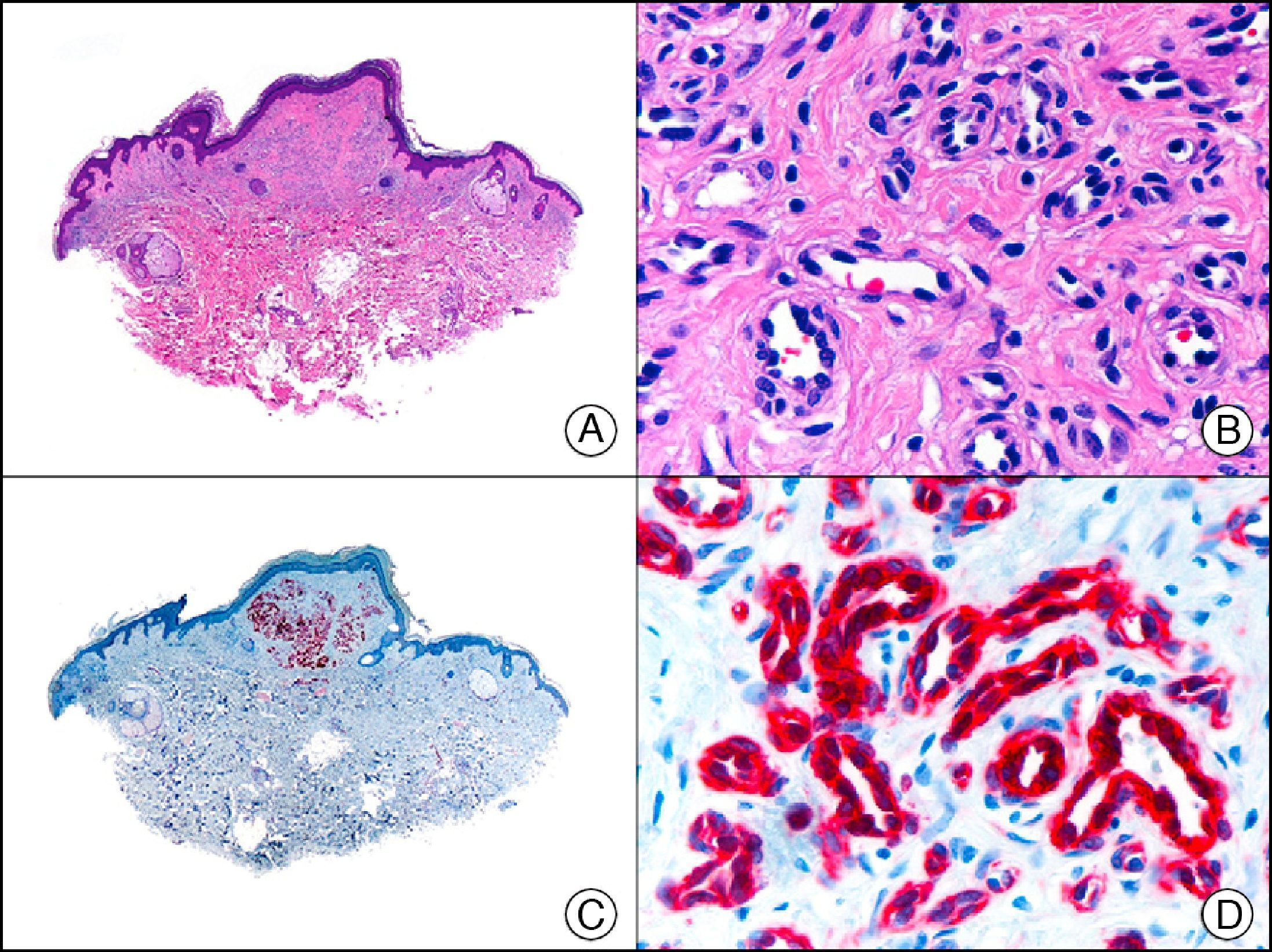
CD34 is a sialomucin whose synthesis is encoded by a gene located at chromosome 1q32. It is expressed in endothelial cells, hematopoietic precursor cells, and dendritic fibroblasts. It is used mainly as an endothelial marker and in the differential diagnosis of soft tissue tumors. It is expressed in 90% of benign and malignant endothelial tumors and is a highly sensitive marker of neoplastic cells in Kaposi sarcoma; it is therefore of value in differentiating this entity from pseudo-Kaposi lesions in acroangiodermatitis secondary to chronic venous insufficiency of the lower limbs.33 CD34, however, is not expressed exclusively in endothelial cell proliferations. It has also been observed in neoplastic cells in epithelioid sarcoma, dermatofibrosarcoma protuberans (helping this to be distinguished from dermatofibroma), plaque-like dermatofibroma (formerly known as Medallion-like dermal dendrocyte hamartoma) (Fig. 23), leiomyosarcoma, spindle cell lipoma, solitary fibrous tumor, fibrous papule of the nose, neurofibroma, extra-abdominal fibromatosis, and many other nonendothelial tumors.34
Plaque-like dermatofibroma. A, Low-power magnification of a lesion extending horizontally into the superficial dermis (×10). B, High-power magnification showing that the lesion is formed by spindle cells arranged in a storiform pattern around the vessels (×200). C, The same sample studied immunohistochemically with CD34 (×10). D, Detail of CD34-positive neoplastic cells (×200).
In dermatopathology, it has also been proposed as a marker for differentiating between trichoblastoma and basal cell carcinoma as it is positive in stromal fibrocytes in trichoblastoma but not in basal cell carcinoma.35–38 Several proliferations with differentiation towards the outer root sheath of the hair follicle, such as trichilemmoma,39,40 desmoplastic trichilemmoma,39,41 trichilemmal keratosis,42 and proliferating trichilemmal tumor40 have also shown highly variable levels of CD34 reactivity in proliferating epithelial cells. Staining is negative in other adnexal skin tumors and in most epithelial tumors.1
The monoclonal WT-1 (Wilms tumor 1) antibody is used to identify a transcription factor encoded by a gene at chromosome 11p13. This transcription factor is involved in regulating the transcription of other genes and may also act as a transcription activator or repressor. The antibody stains the nucleus of normal cells of highly diverse origins, such as glomerular epithelial cells, developing gonadal cells, Sertoli cells, epithelial and granulosa cells of the ovary, and neoplastic cells in very different neoplasms such as leukemia, lung cancer, colorectal cancer, breast cancer, brain tumors, and desmoid tumors. In dermatopathology, it is a very useful marker for distinguishing vascular tumors from vascular malformations,43 as it is expressed in the proliferating endothelium stimulated by angiopoietin 2 in vascular hyperplasia and neoplasms (Fig. 24). Nevertheless, it is negative in nonproliferating endothelial cells of vascular malformations as defects in WT-1 signaling indicate that these cells are unable to undergo apoptosis or physiological remodeling.44,45 WT-1 has also been studied in melanocytic proliferations, with recent findings indicating that it is expressed in the cytoplasm of proliferating melanocytes in both melanocytic nevi and melanoma, although staining is more intense in more advanced cases of melanoma, indicating that WT-1-positive melanocytic tumors are associated with poor prognosis.46
Lobular capillary hemangioma. A, Low-power magnification (×10). B, Detail of lesion showing capillary vessels lined by endothelial cells (×400). C, The same sample studied immunohistochemically with WT-1 (Wilms tumor 1) (×10). D, High-power magnification showing WT-1-positive nuclei in the proliferating endothelial cells (×400).
Podoplanin is a surface sialoglycoprotein that is observed immunohistochemically in different types of cells, including lymphatic endothelial cells, testicular germ cells, follicular dendritic cells, interstitial Cajal cells, myoepithelial cells, and cells in the basal portion of the glandular epithelium. As an endothelial marker, it is expressed in the cytoplasm of lymphatic endothelial cells of healthy tissues and lymphatic malformations (Fig. 25), but positive staining also indicates differentiation towards the lymphatic endothelium in several vascular proliferations of controversial histogenesis, such as Kaposi sarcoma, papillary intralymphatic angioendothelioma (Dabska tumor), epithelioid hemangioendothelioma, and hobnail hemangioma. It is also expressed in the neoplastic cells of the majority of cutaneous angiosarcomas (angiosarcoma of the face and scalp in elderly patients, angiosarcoma secondary to chronic lymphedema or radiation therapy, and epithelioid angiosarcoma). It is also used to demonstrate the lymphatic nature of vessels containing neoplastic cells in the presence of tumor emboli in the lumen of the vessels, both in primary cutaneous tumors and skin metastases. Podoplanin is also useful for differentiating primary cutaneous tumors (in particular, adnexal adenocarcinomas) from skin metastases from internal adenocarcinomas as it is expressed in the neoplastic cells of the former but not of the latter.47,48 Finally, recent studies have proposed using podoplanin, in conjunction with other endothelial markers, such as LYVE-1 (lymphatic vessel endothelial receptor 1) and CD31, to assess lymphatic invasion and predict lymph node metastasis in melanoma.49
Superficial lymphatic malformation. A, Low-power magnification (×10). B, Detail of thin-walled dilated vessels lined with a discontinuous layer of flat endothelial cells occupying the superficial dermis (×200). C, The same sample studied immunohistochemically with podoplanin (×10). Detail of podoplanin expression in the cytoplasm of endothelial cells (×200).
LYVE-1, a lymphatic endothelial nuclear transcription factor, is a hyaluronan receptor expressed almost exclusively in the lymphatic endothelium. While its function has not yet been fully elucidated, LYVE-1 appears to have a role in hyaluronan transport and turnover or in the localization of this hyaluronan to the surface of the lymphatic endothelium. It is expressed on both the luminal and abluminal surface of the lymphatic endothelium and in the hepatic sinusoids. Several studies have demonstrated its ability to identify lymphangiogenesis (mainly in inflammatory diseases of the cornea and skin) in addition to lymph tissue. It has also been used as a marker of lymphangiogenesis in different tumors, meaning that it has prognostic in addition to diagnostic value.49 Its use in dermatopathology is similar to that of podoplanin (Fig. 26), although it is less specific as it is also expressed in macrophages and adipocytes. A loss of staining intensity has been observed for both LYVE-1 and podoplanin in the endothelium of large lymph vessels.50
Kaposi sarcoma, nodular stage. A, Low-power magnification (×10). B, High-power magnification of fascicles of spindle cells and small vascular spaces filled with red blood cells (×200). C, The same sample studied immunohistochemically with LYVE-1 (lymphatic vessel endothelial receptor 1) (×10). D, Detail showing LYVE-1-positive neoplastic cells (×200).
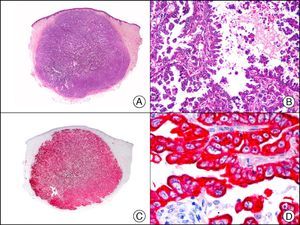
PROX-1 (prospero-related homeobox 1) is a nuclear transcription factor that plays an essential role in embryonic lymphangiogenesis. As an immunostain, it is very useful for differentiating between lymphatic and blood endothelial cells, because in healthy skin and mucosa, it specifically labels the nucleus of lymphatic but not blood endothelial cells (Fig. 27). Although PROX-1 is highly specific for lymphatic endothelium, it is also expressed in nonendothelial cells, such as hepatocytes and central nervous system cells. It is therefore advisable to use it in combination with other endothelial markers, such as CD31 and VEGFR-3 (vascular endothelial growth factor 3).51 This marker is therefore used in diagnostic immunopanels for the investigation of a large number of vascular tumors and malformations.52,53
Intralymphatic histiocytosis. A, Low-power magnification (×10). B, Detail showing clusters of intravascular histiocytes (×200). C, The same sample studied immunohistochemically with PROX-1 (prospero-related homeobox 1) (×10). D, High-power magnification showing PROX-1 expression in nuclei of cells lining the vessels that contain histiocytes (×200). This positive staining demonstrates the lymphatic nature of the vessels.
VEGFR-3 is initially expressed in embryonic blood vessels but is then limited almost exclusively to lymph vessels.53 It has relatively good specificity for lymphatic endothelium, but performs less well than LYVE-1, or PROX-1, as it is also expressed in the endothelium of some proliferating blood vessels.54 VEGFR-3, however, has high sensitivity for endothelial cells, and produces similar results to PROX-1 and better results than podoplanin.55
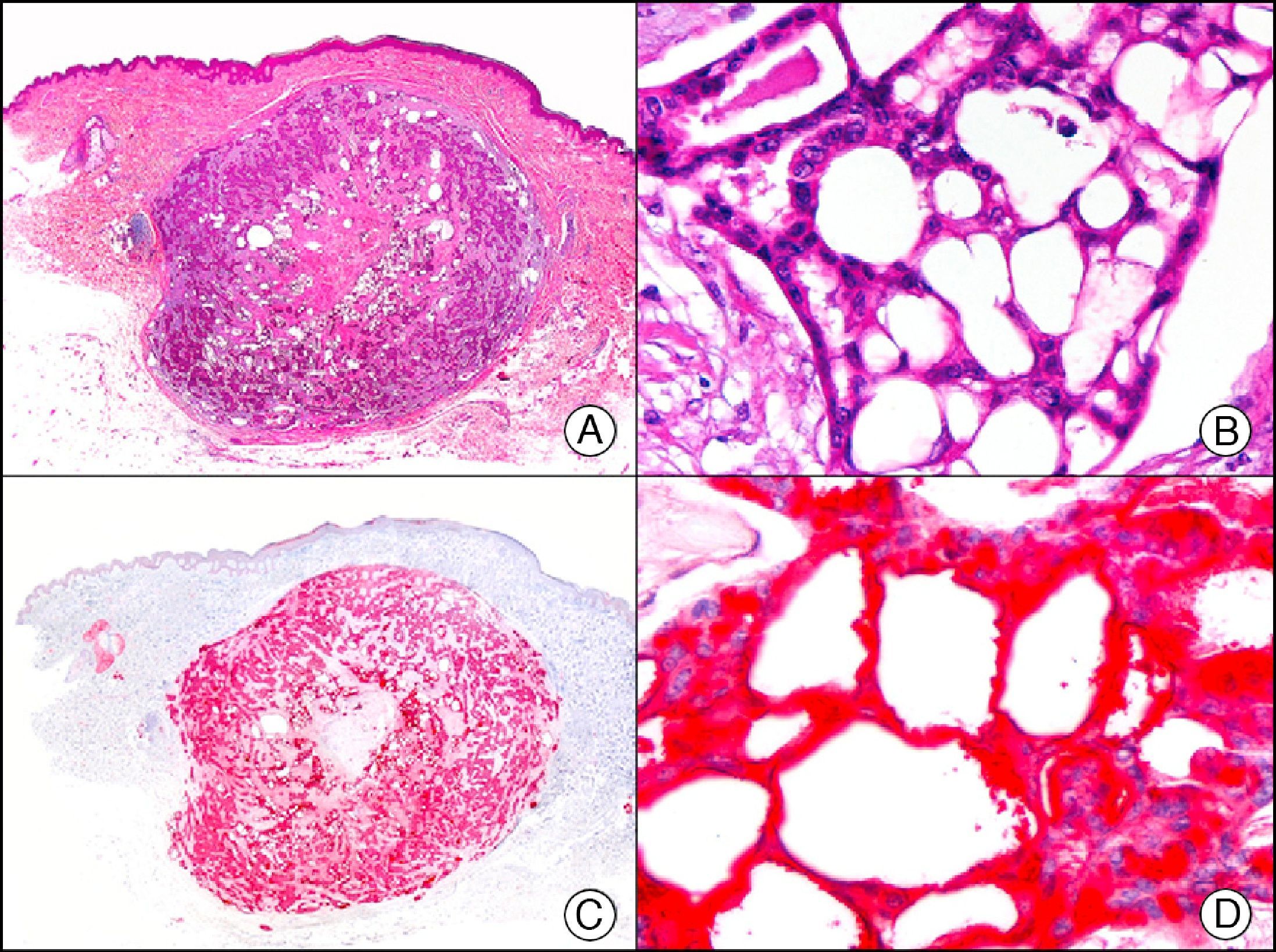
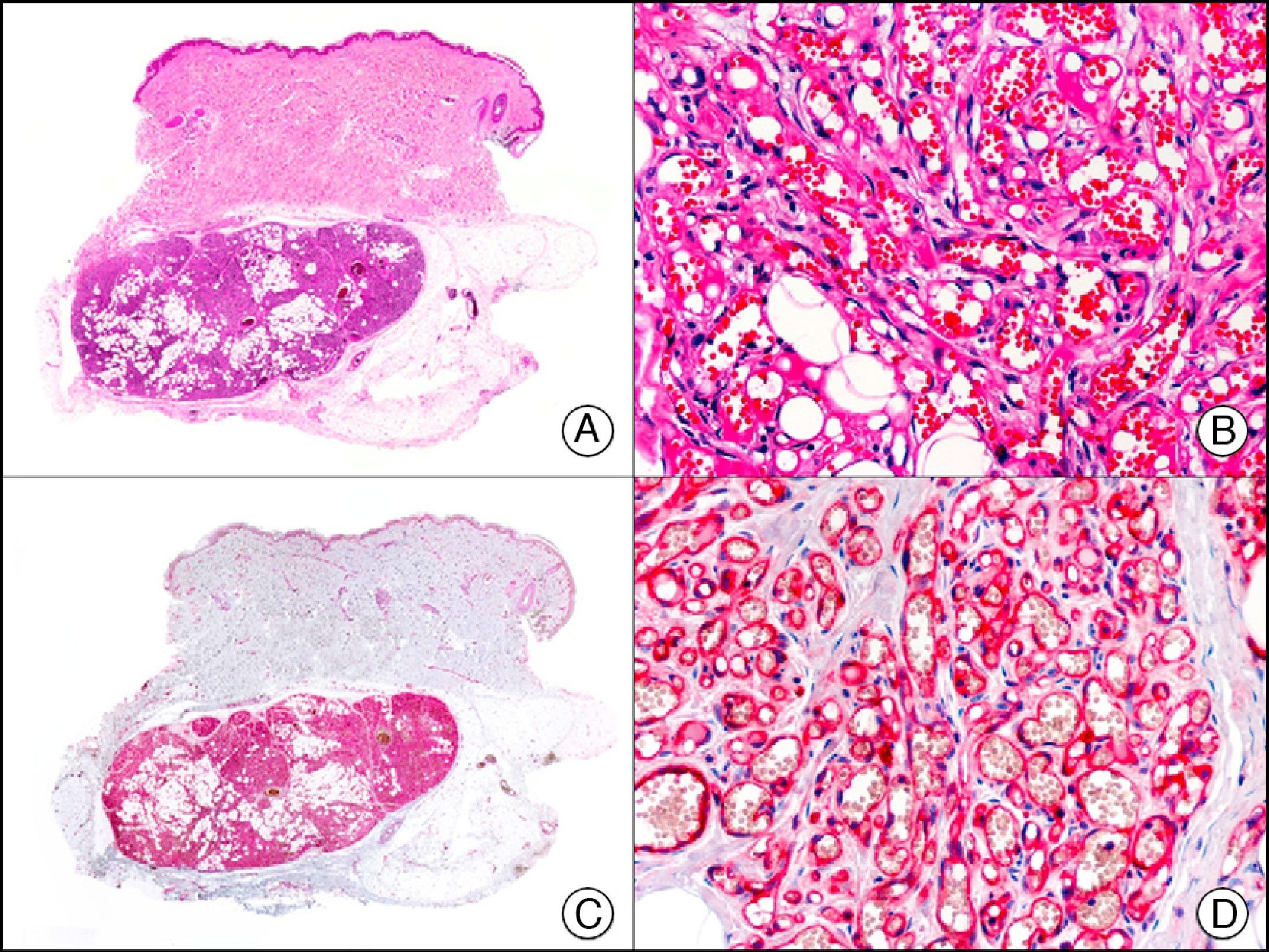
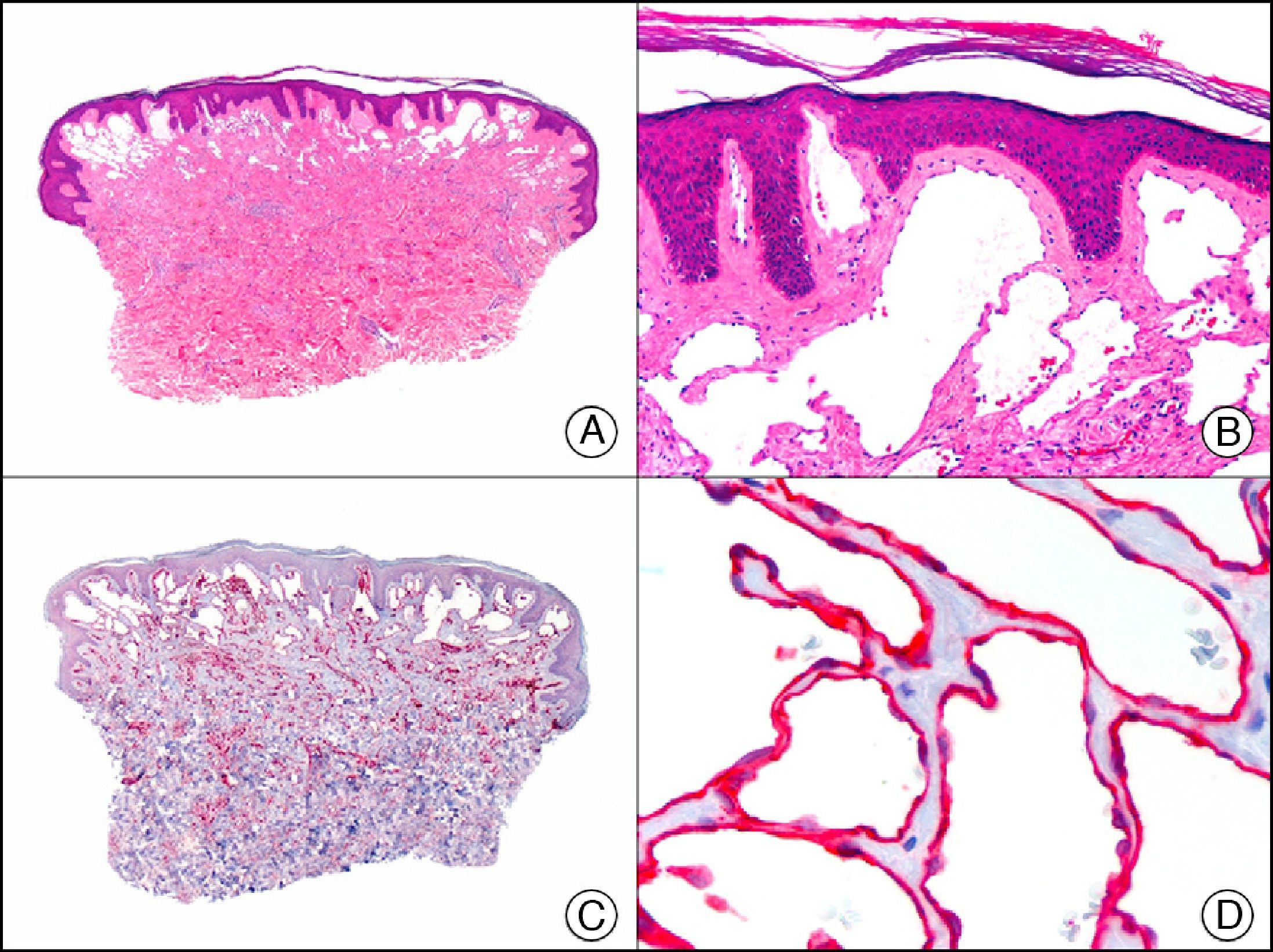
GLUT-1 (glucose transporter 1) antibody, which is similar to the glucose transporter in red blood cells, stains the cytoplasmic membrane of endothelial cells in certain blood-tissue barriers, such as the blood-brain barrier and placenta. It is particularly useful in the identification of proliferating endothelial cells in infantile hemangiomas (Fig. 28), and specifically labels these cells throughout their development stages. It is, however, absent in the endothelium of vascular malformations and in several vascular proliferations, such as pyogenic granulomas and granulation tissue. It is also expressed in the endothelium of several rare infantile hemangiomas, of both the rapidly involuting and noninvoluting type.3 It is noteworthy that GLUT-1 is an excellent marker of neoplastic cells in cutaneous perineurioma.
Infantile hemangioma. A, Low-power magnification (×10). B, High-power magnification of proliferating endothelial cells (×400). C, The same sample studied immunohistochemically with GLUT-1 (glucose transporter 1) (×10). D, Detail of GLUT-1-positive endothelial cells lining the lumen of the newly formed capillaries (×400).
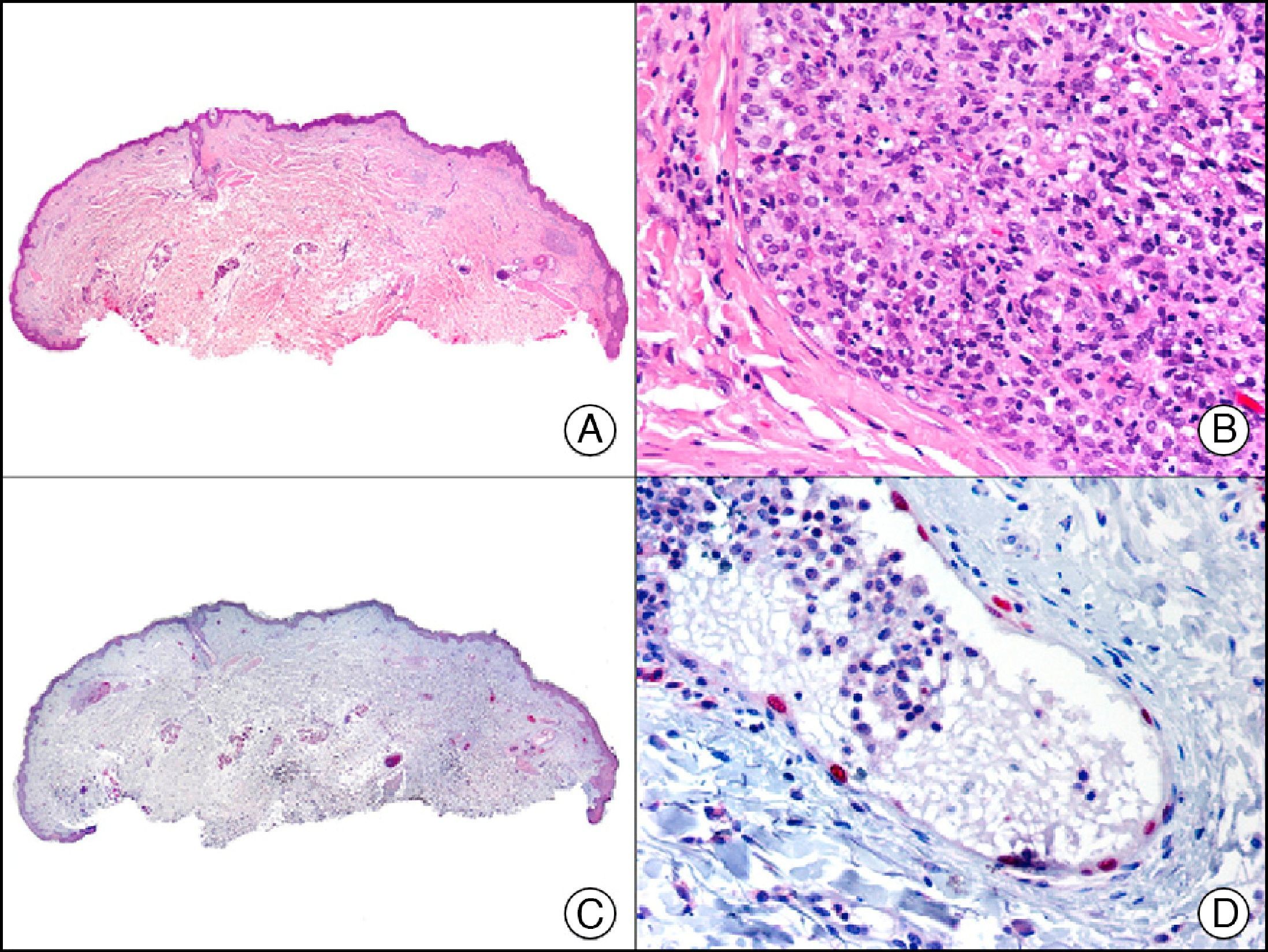
FLI-1 (friend leukemia virus integration 1) protein initially emerged as a diagnostic marker for Ewing sarcoma and other primitive neuroectodermal tumors. In healthy tissue it recognizes endothelial cells and small lymphocytes (possibly T cells). Like PROX-1, it offers an advantage over other endothelial markers in that it is a nuclear marker and is therefore easier to interpret. It is also very sensitive in the identification of proliferating cells in hemangiomas, hemangioendotheliomas, angiosarcomas, and Kaposi sarcomas. However, like other endothelial markers, it is unable to distinguish between benign and malignant vascular proliferations. A negative result, however, can be useful, as FLI-1 is not expressed in neoplastic cells in nonvascular sarcomas, melanomas, or carcinomas. Because FLI-1 is expressed in lymphocytes, when interpreting results, care should be taken not to confuse lymphocytes with tumor cells.3
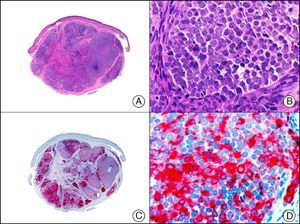
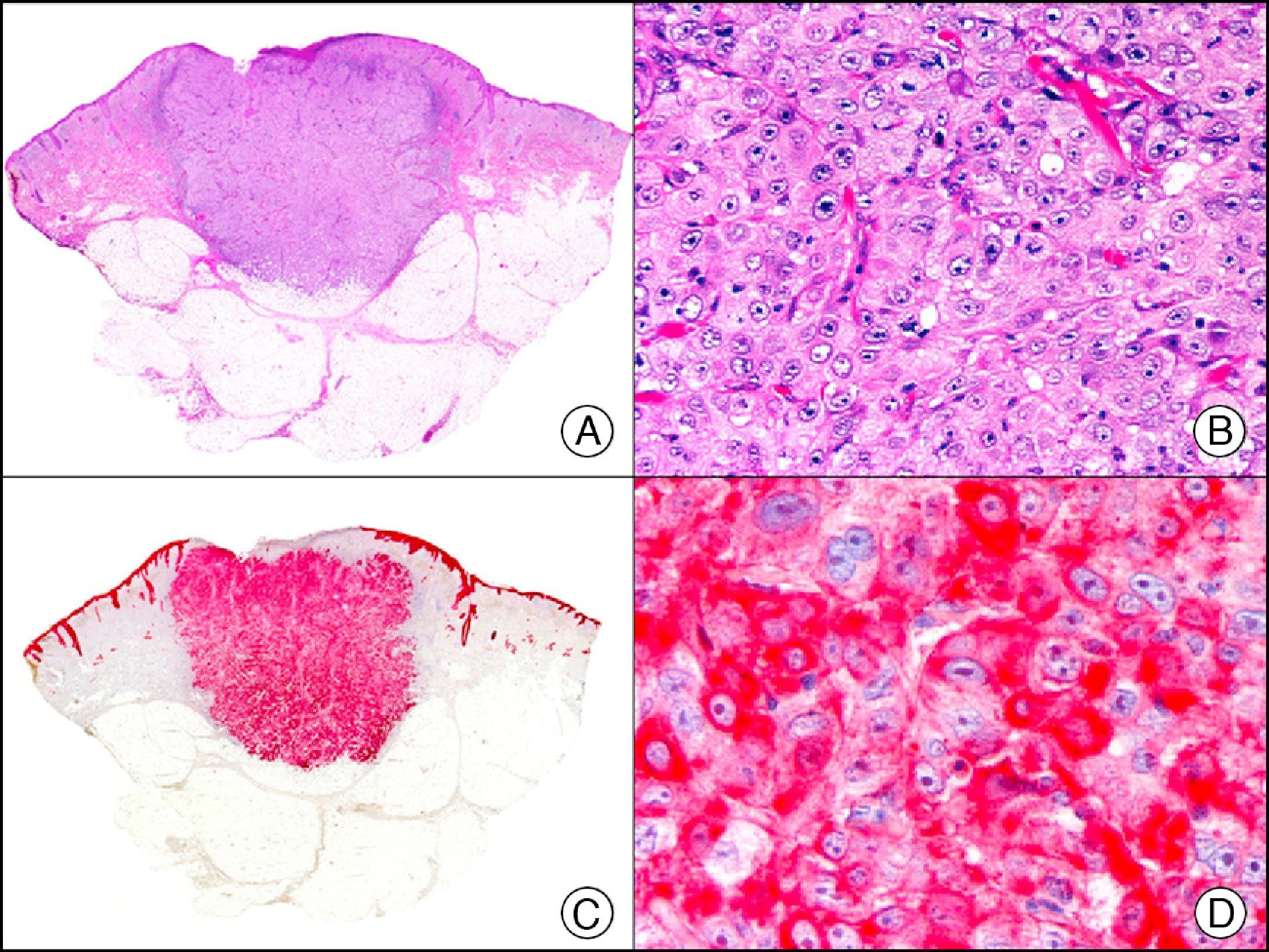
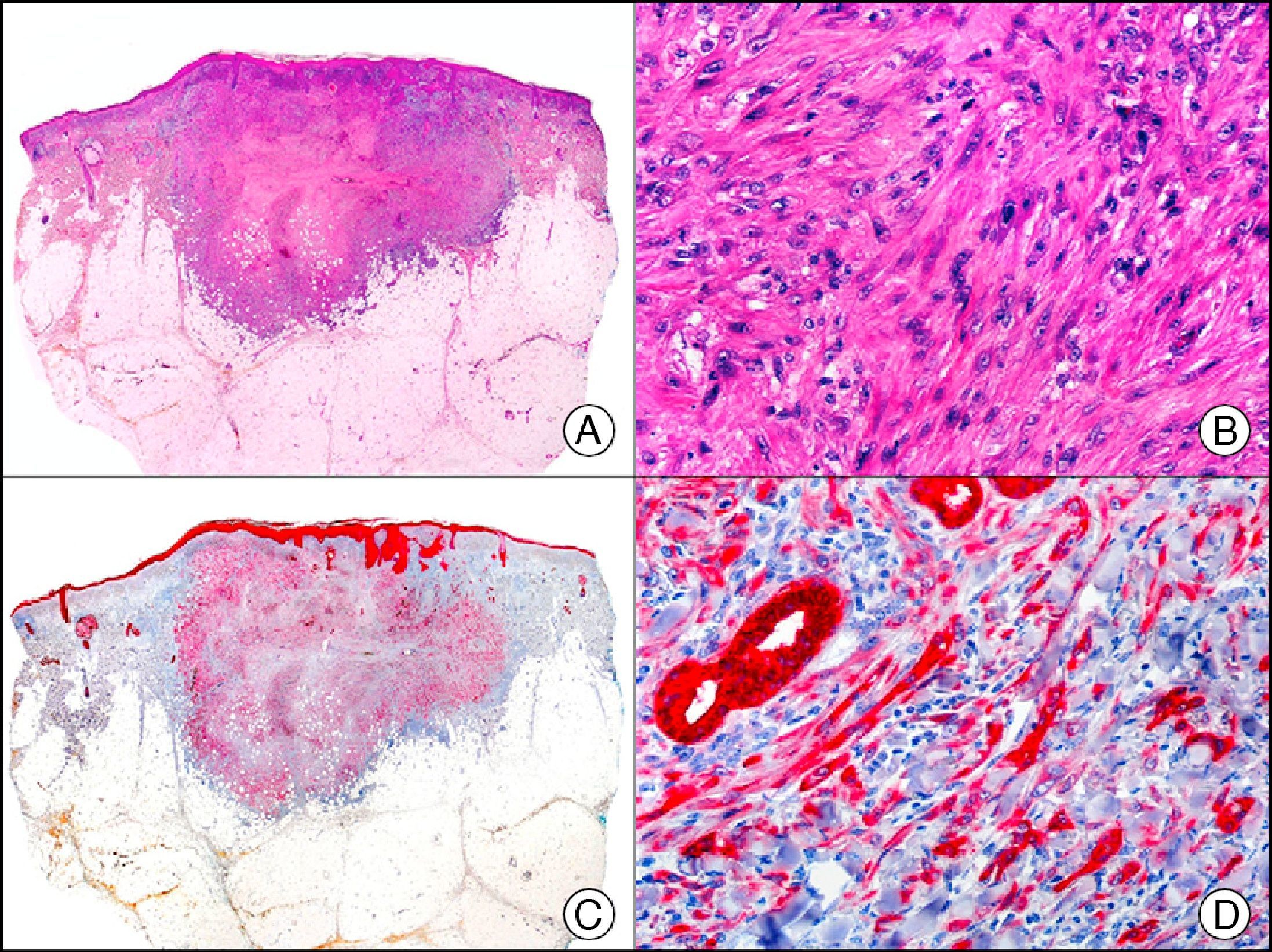
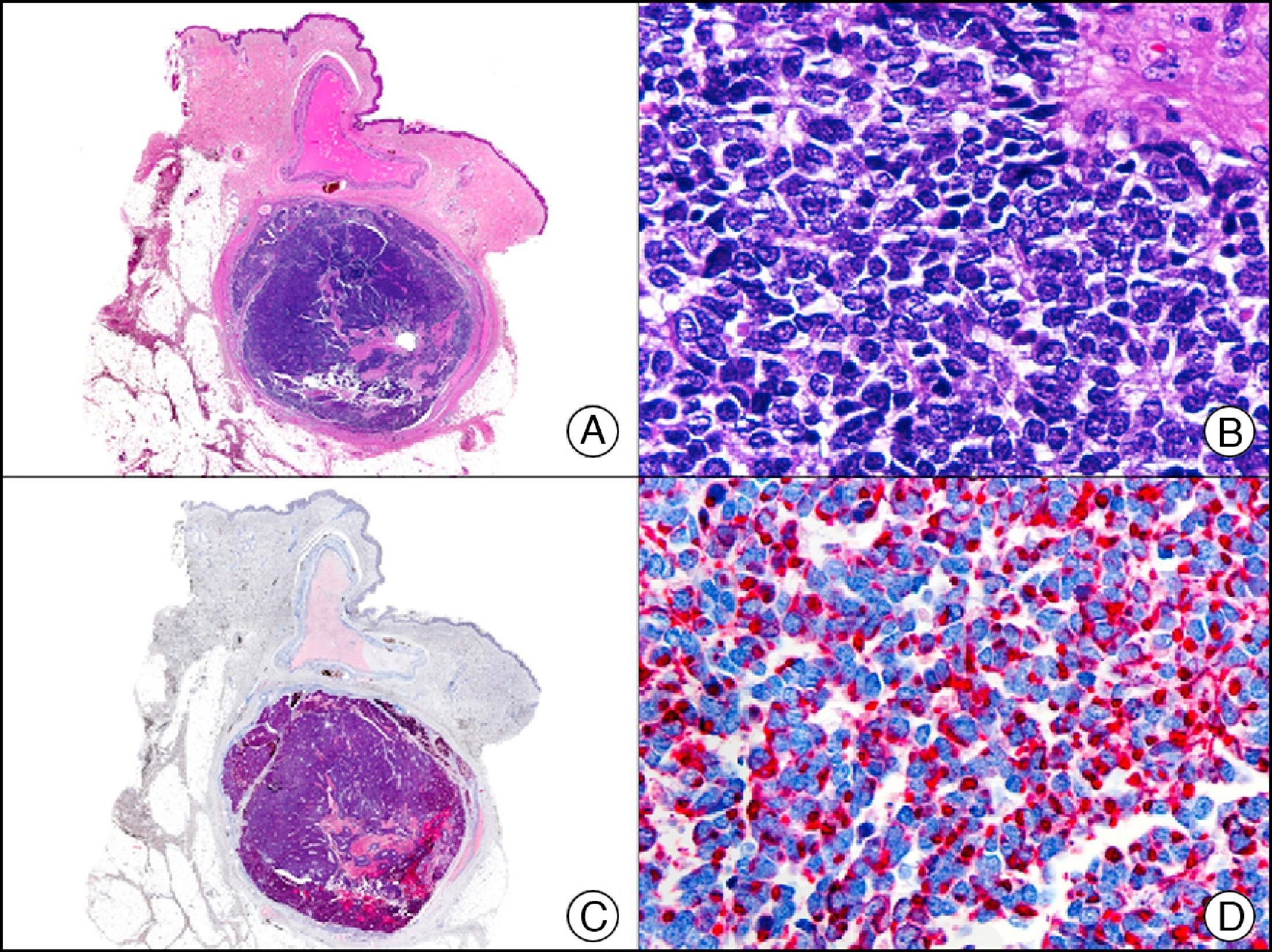
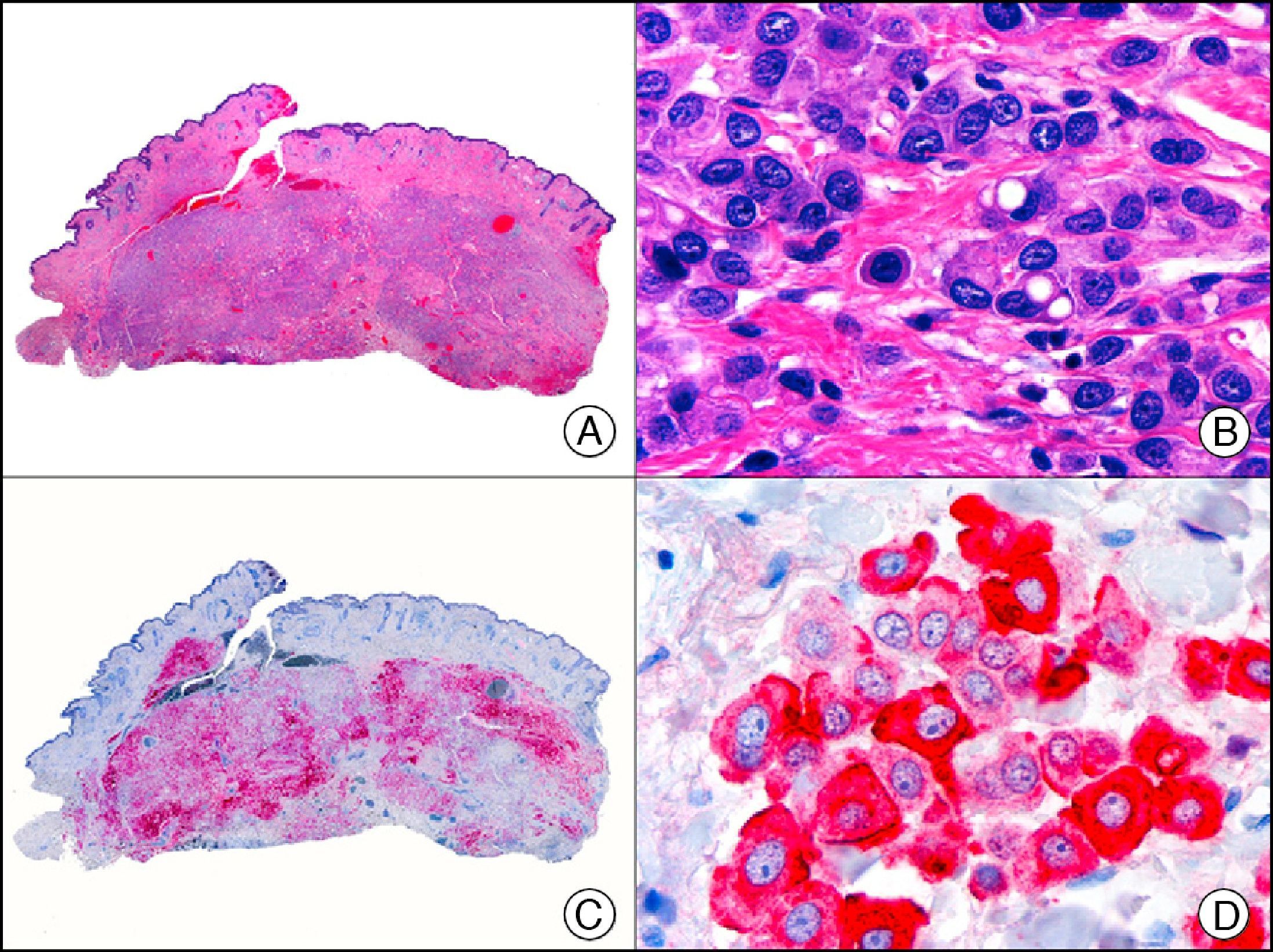
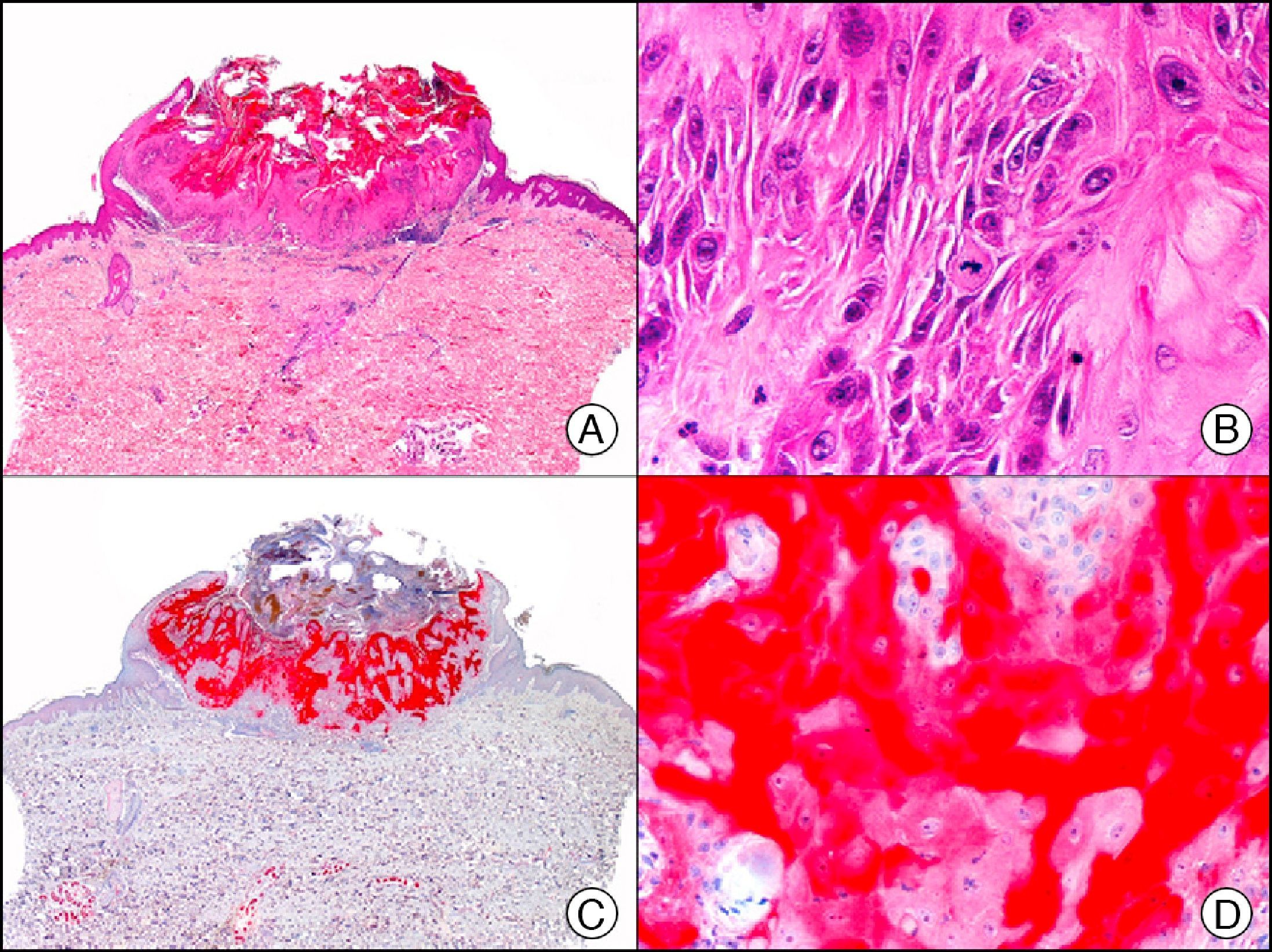
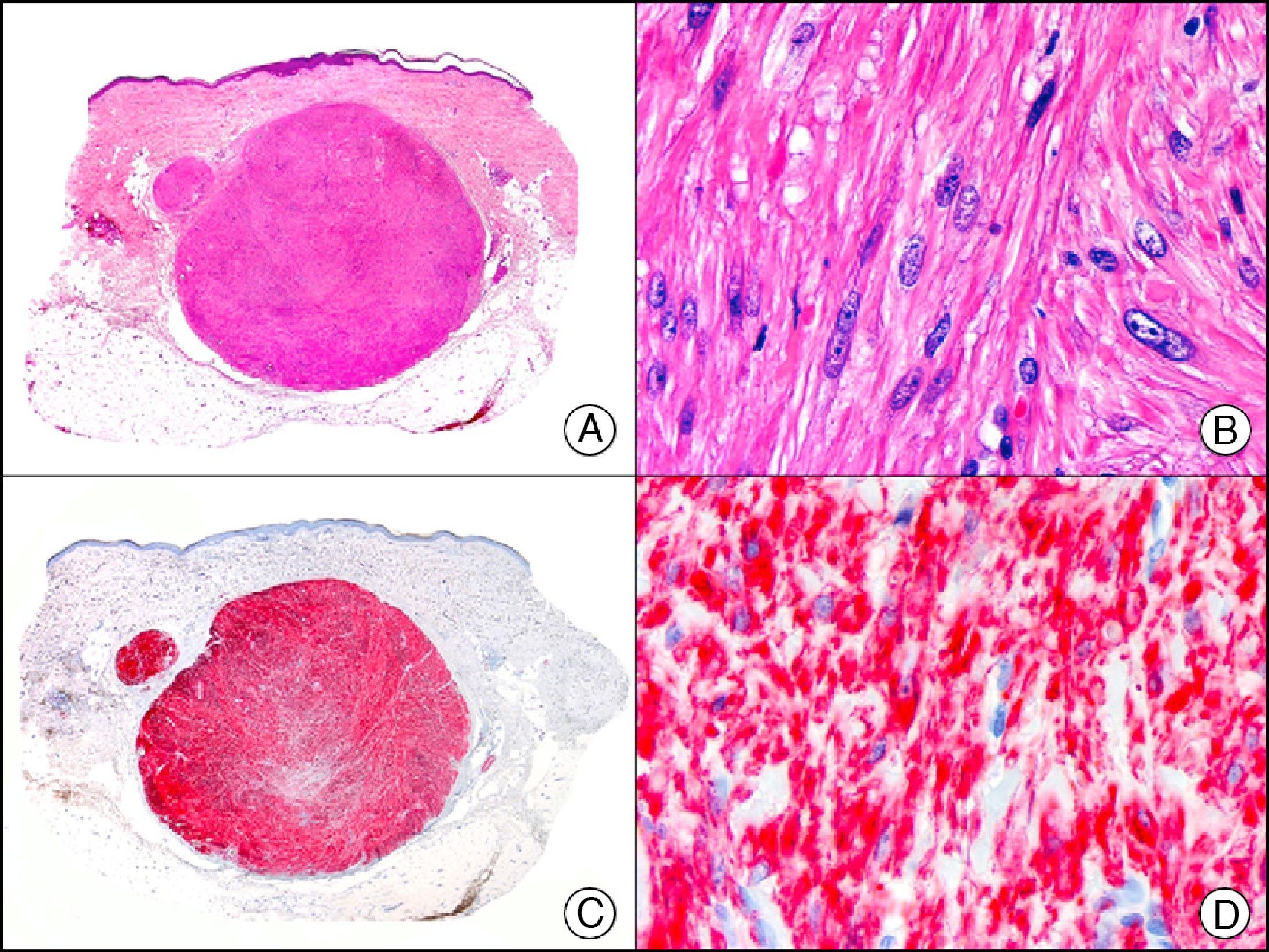
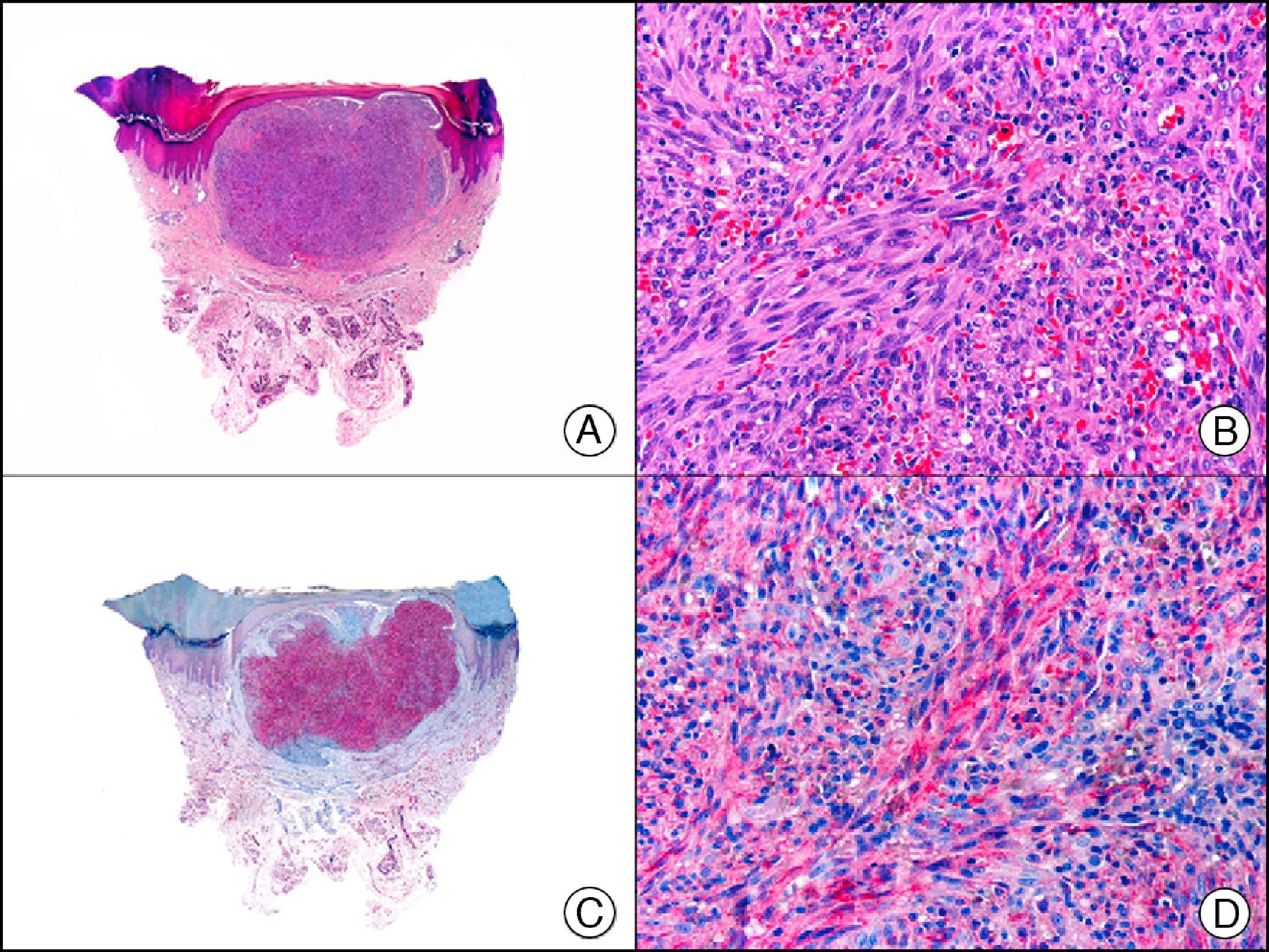
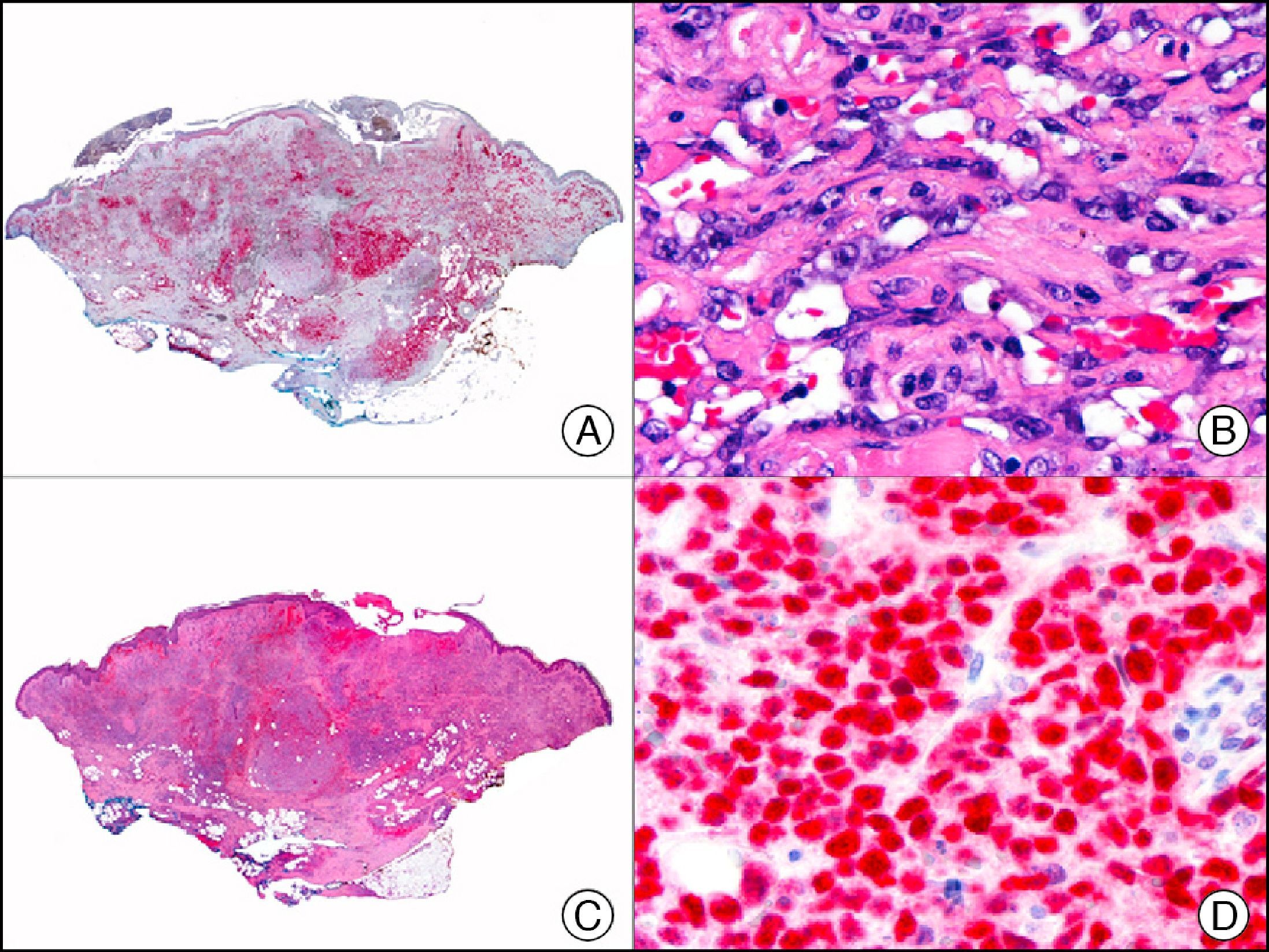
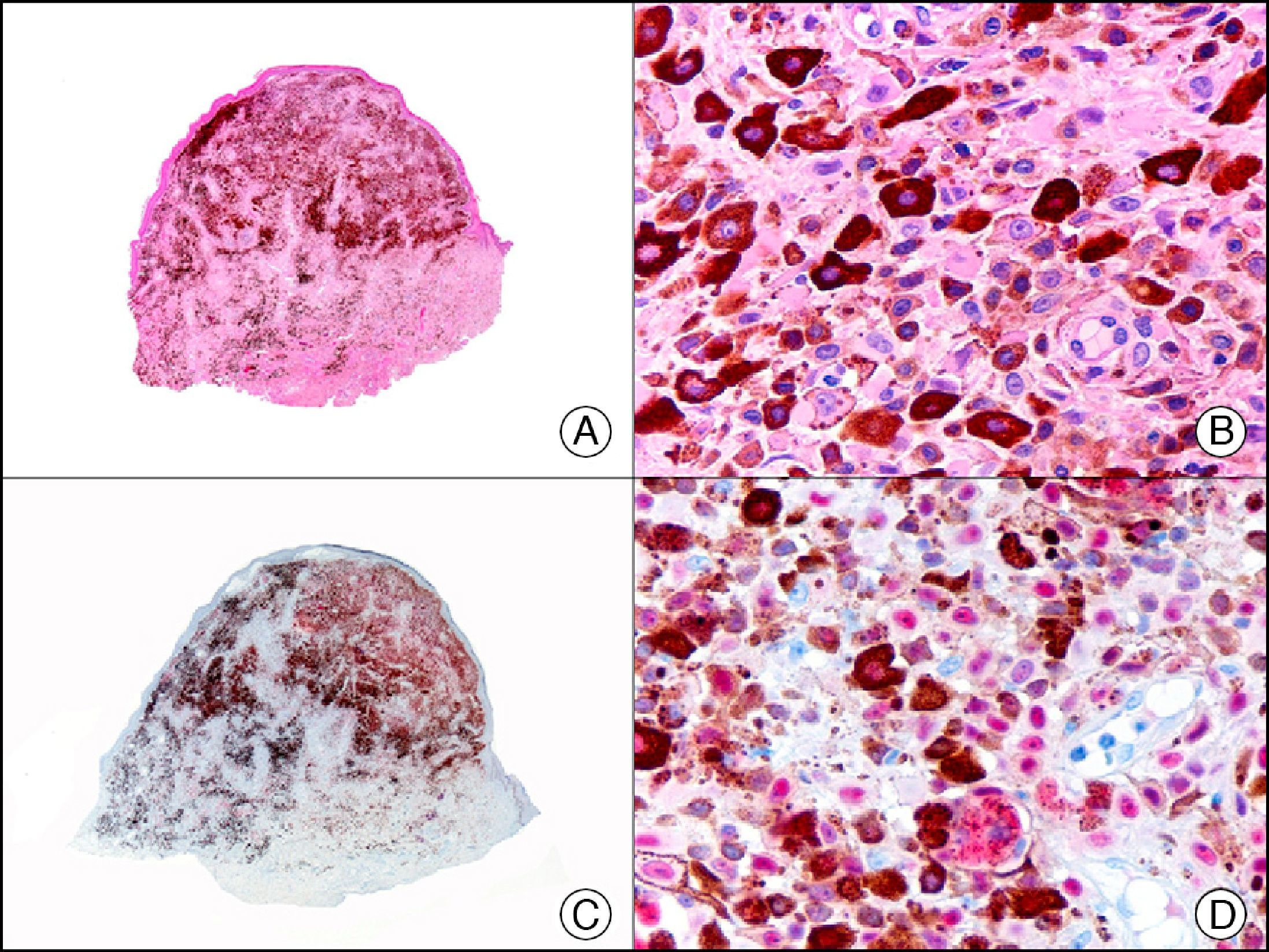
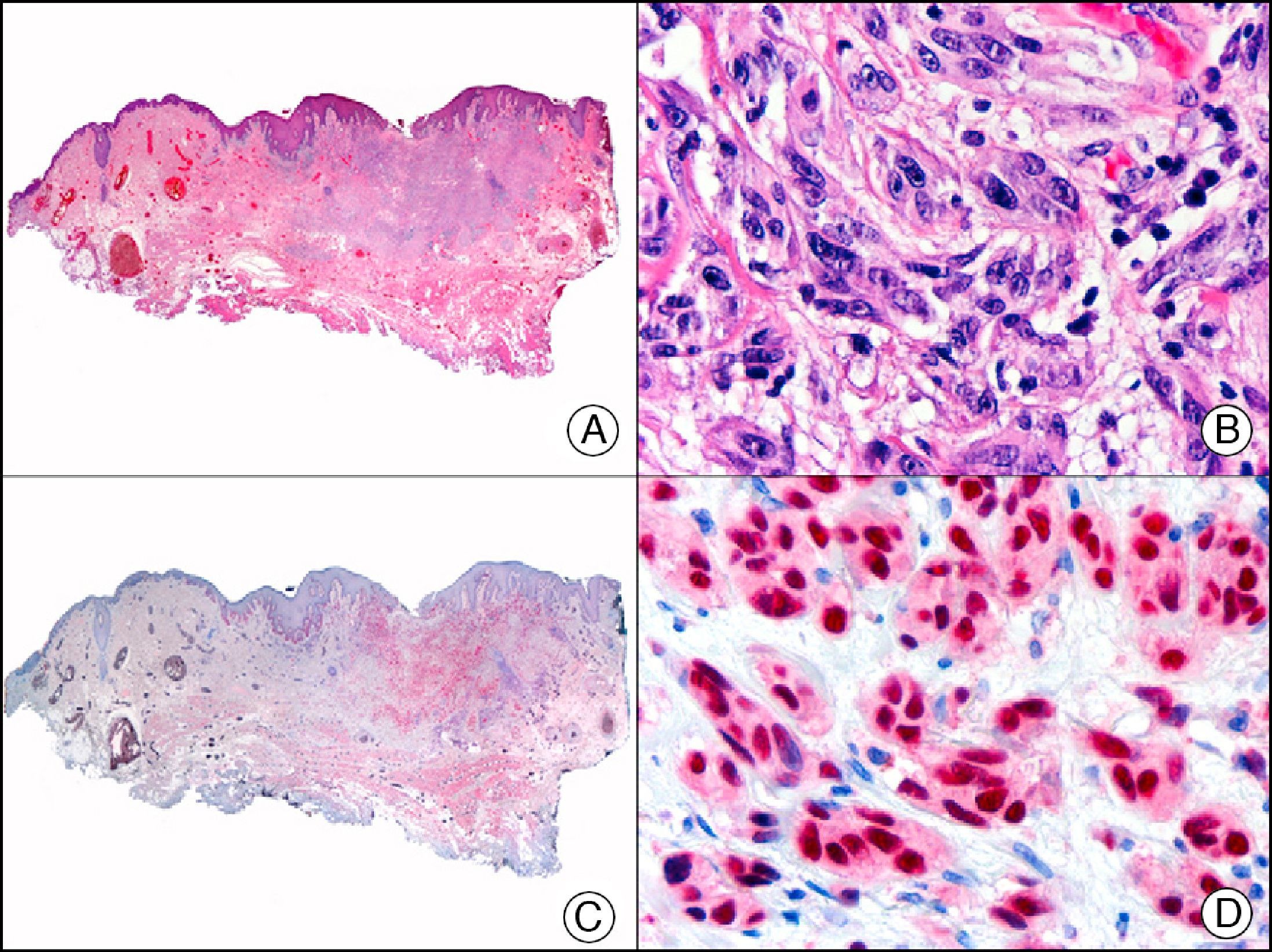
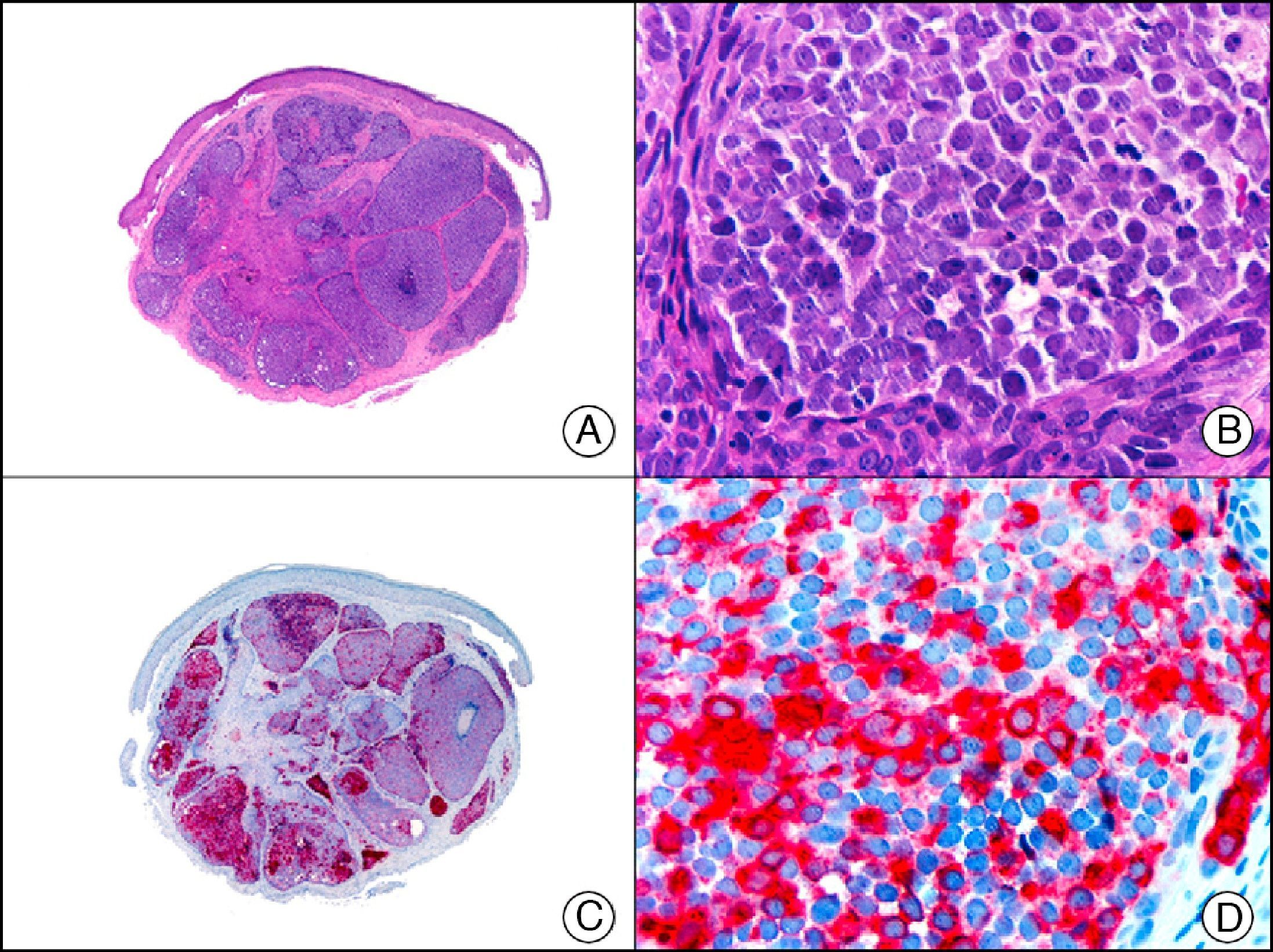
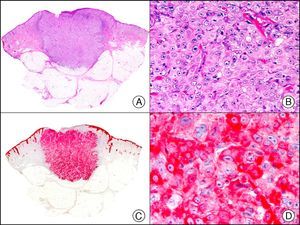
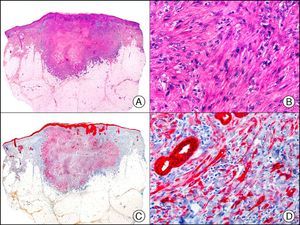
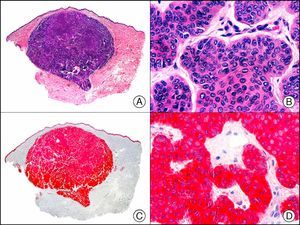
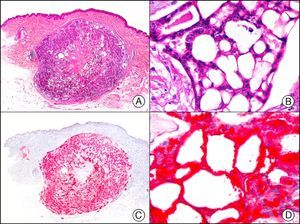
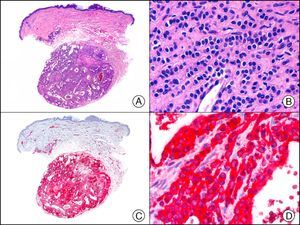
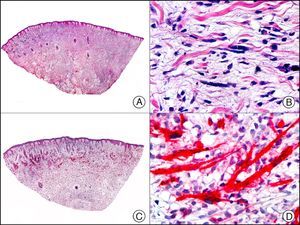
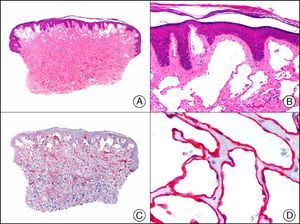
For years, researchers searched in vain for chromosomal aberrations in angiosarcomas. However, it was recently discovered that angiosarcomas associated with chronic lymphedema or radiation therapy present relatively recurrent genetic alterations that are more common than in angiosarcoma of the face and scalp in elderly patients (Wilson-Jones angiosarcoma). Gene amplifications at chromosomes 8q24.21 (50%), 10p12.33 (33%), and 5q35.3 (11%) are the most common genetic alterations. Amplification of V-myc myelocytomatosis viral oncogene homolog (avian) (c-Myc) at chromosome 8q24.21 is the most frequently observed alteration, present in over 50% of angiosarcomas secondary to radiotherapy and chronic lymphedema. This has important implications for the diagnosis, and probably in the near future, the treatment of these tumors.56 c-Myc amplification was initially studied by fluorescent in situ hybridization but a commercial antibody is now available for the immunohistochemical analysis of this event in formalin-fixed and even paraffin-embedded tissue (Fig. 29). c-Myc amplification is not detected in atypical vascular proliferations in benign tumors induced by radiation therapy. This has important implications as these tumors are histologically very difficult to differentiate from radiation-induced angiosarcomas. Finally, it is important to note that c-Myc amplification is not exclusive to angiosarcomas: it has also been detected in Kaposi sarcoma, malignant fibrous histiocytoma of the bone, high-grade chondrosarcoma, and methotrexate-resistant osteosarcoma.57
Angiosarcoma of the breast skin following radiation therapy for breast cancer. A, Low-power magnification (×10). B, Irregular vessels lined with atypical endothelial cells (×400). C, The same sample studied immunohistochemically for amplification of c-Myc (V-myc myelocytomatosis viral oncogene homolog [avian])(×10). D, Most of the nuclei of the neoplastic cells are strongly positive for c-Myc (×400).
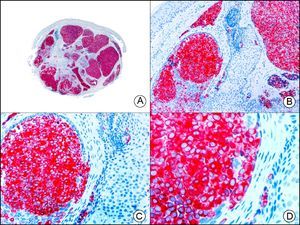
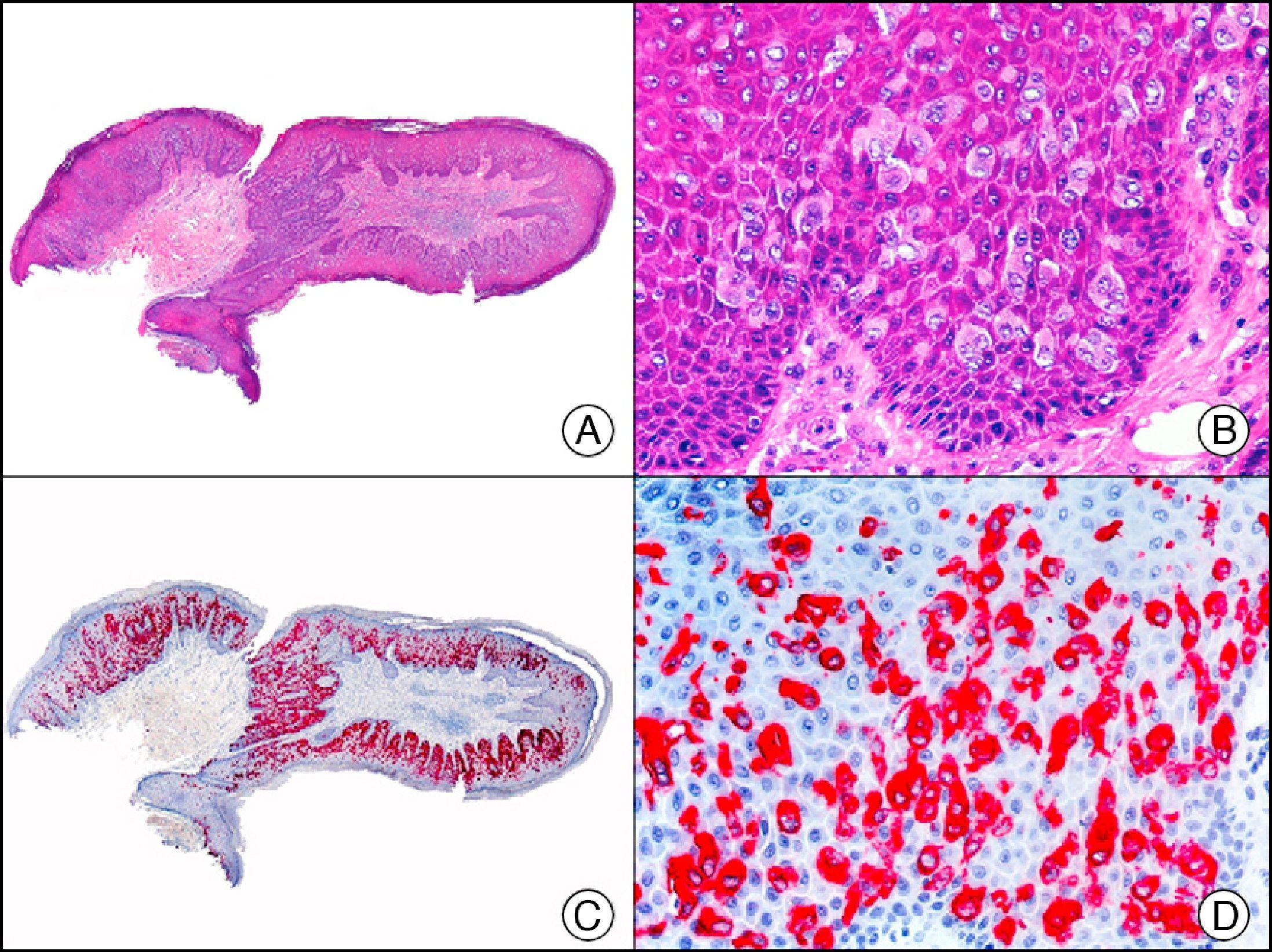
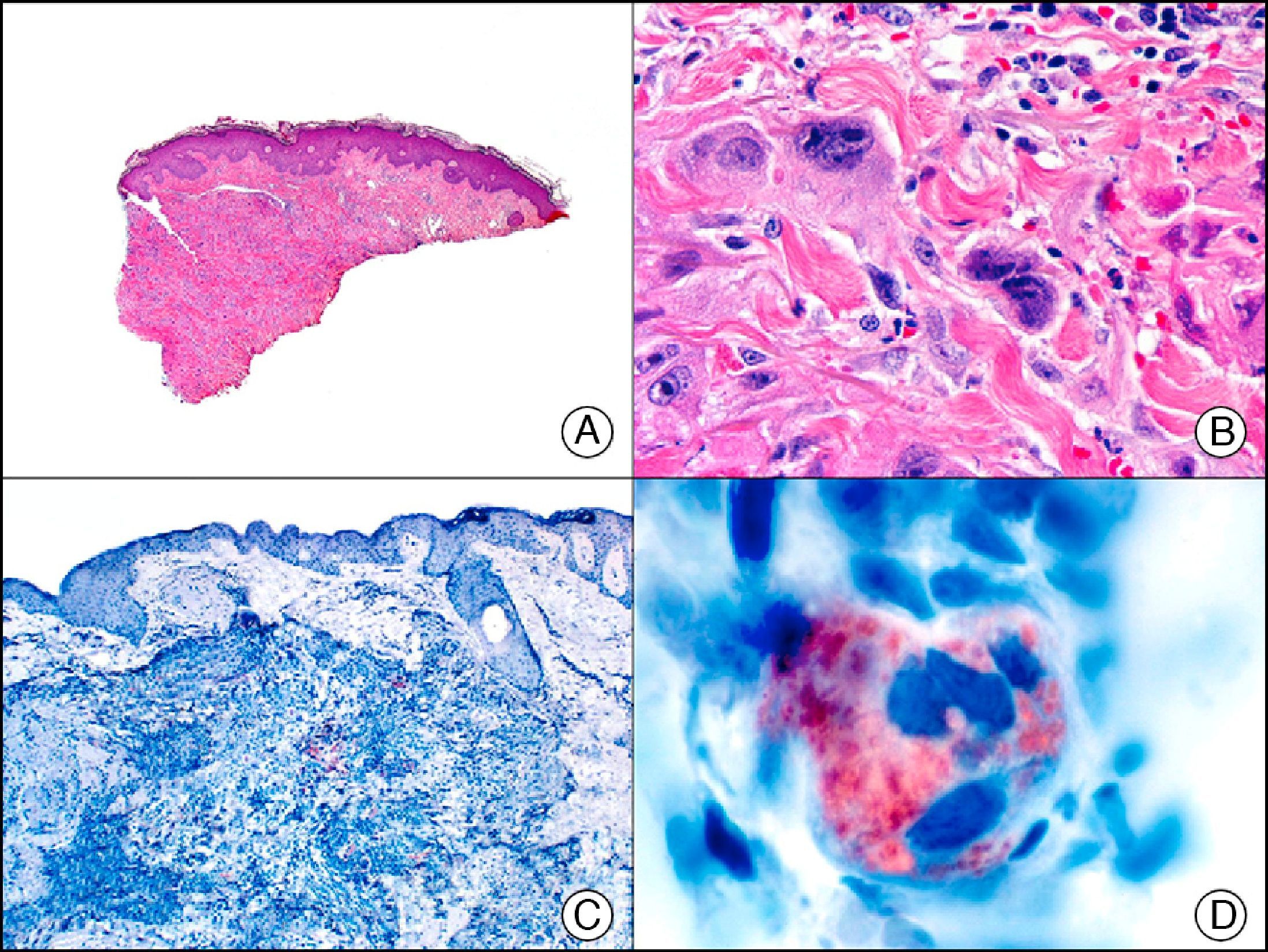
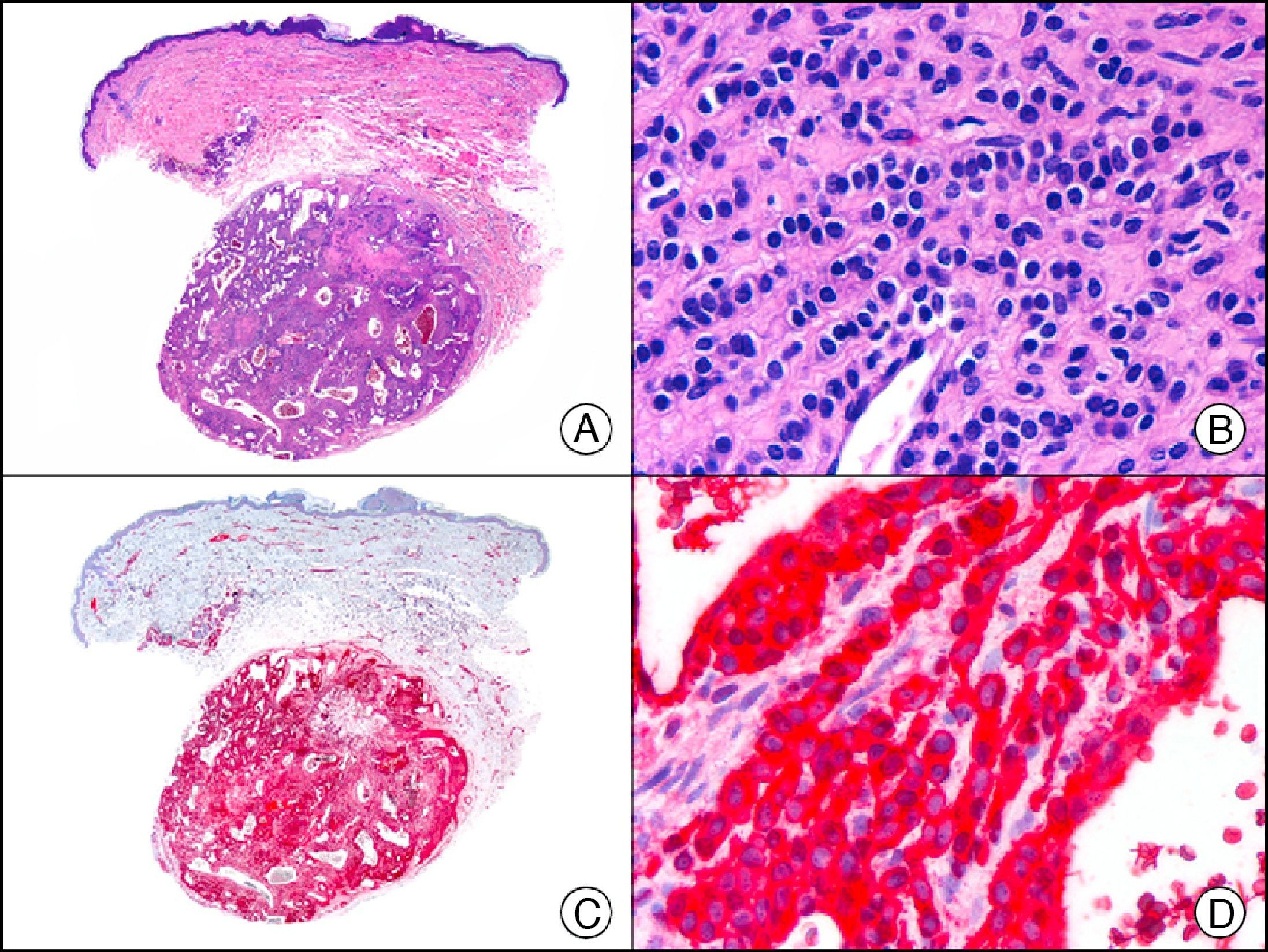
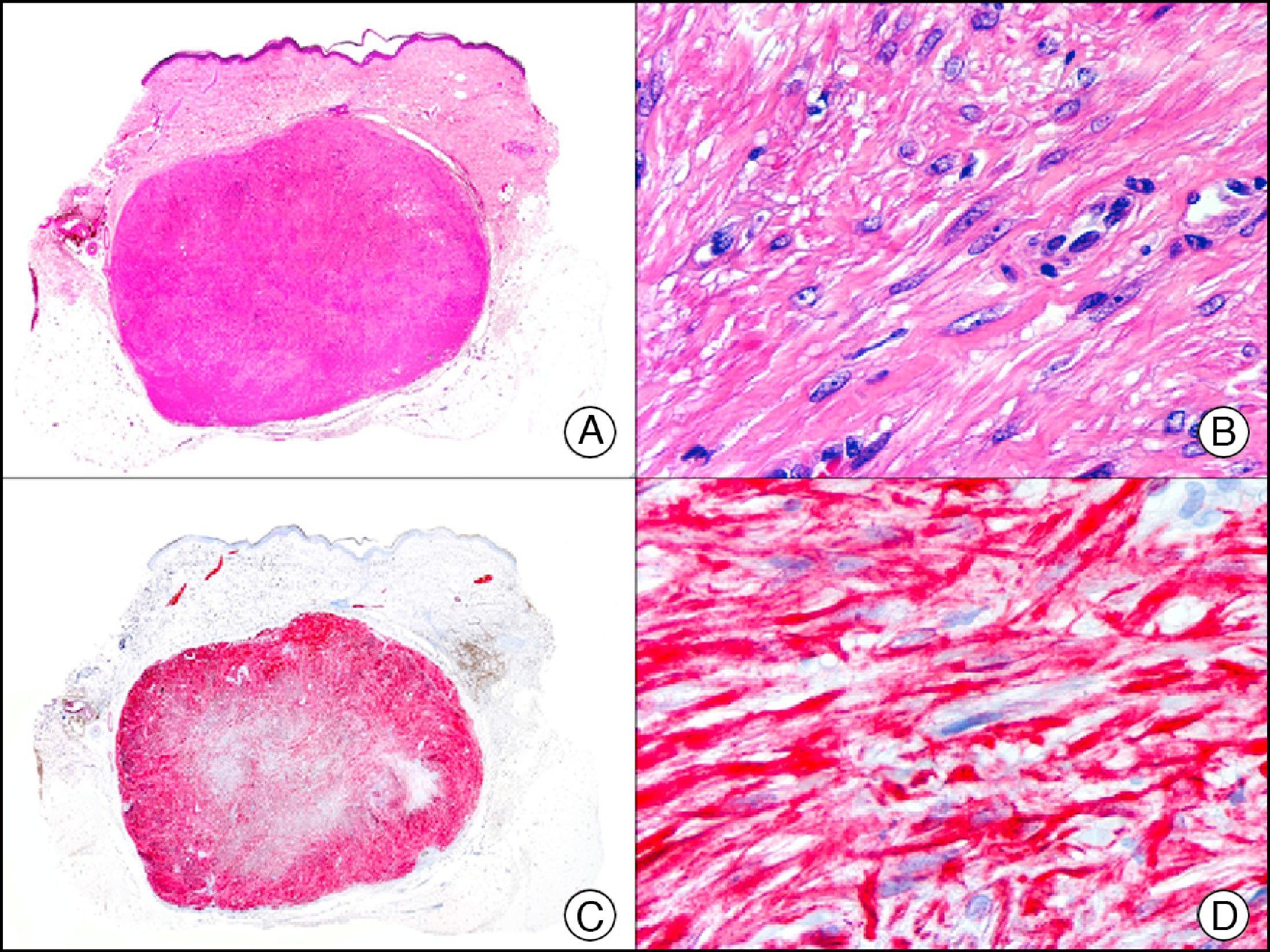
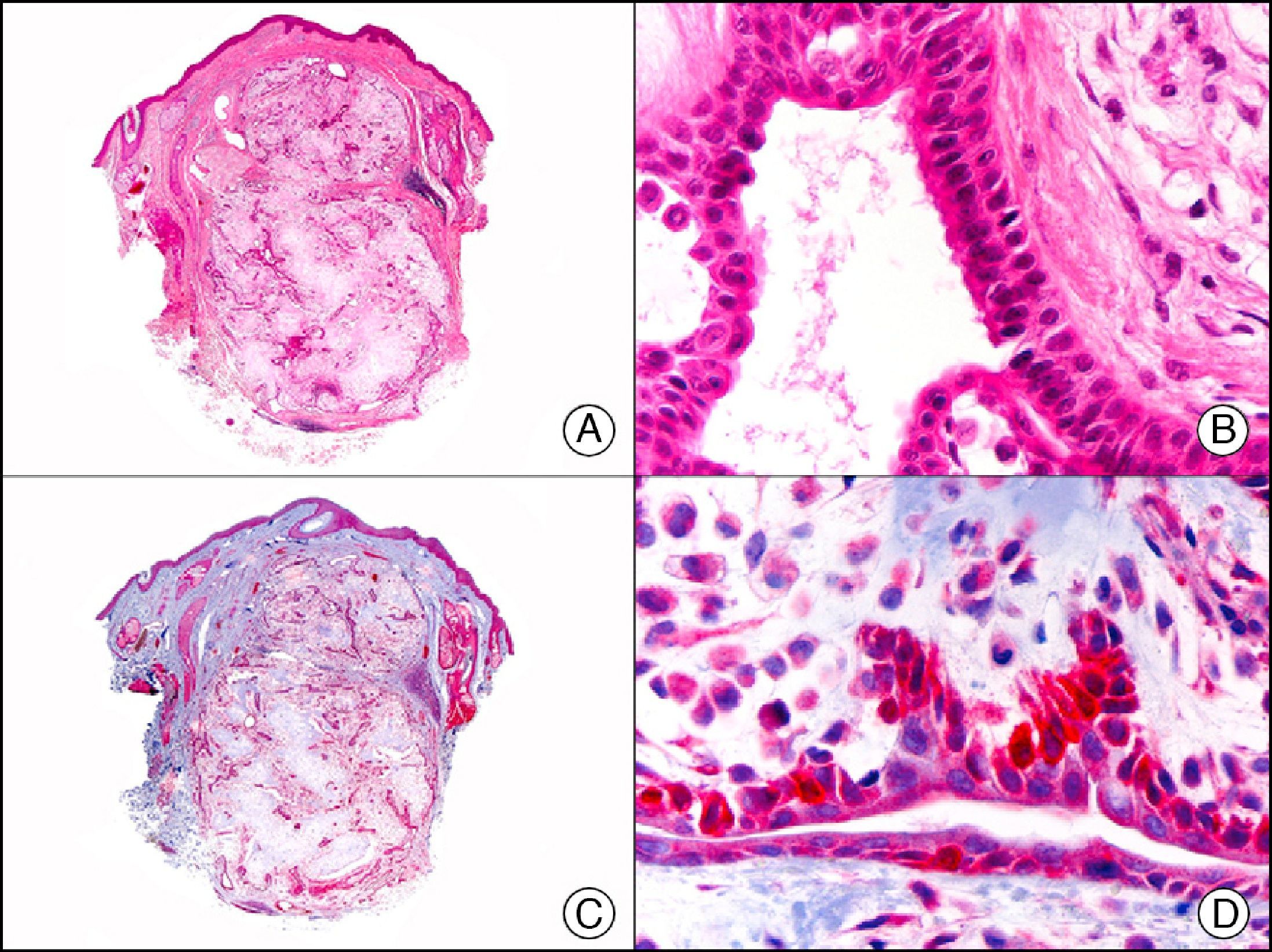
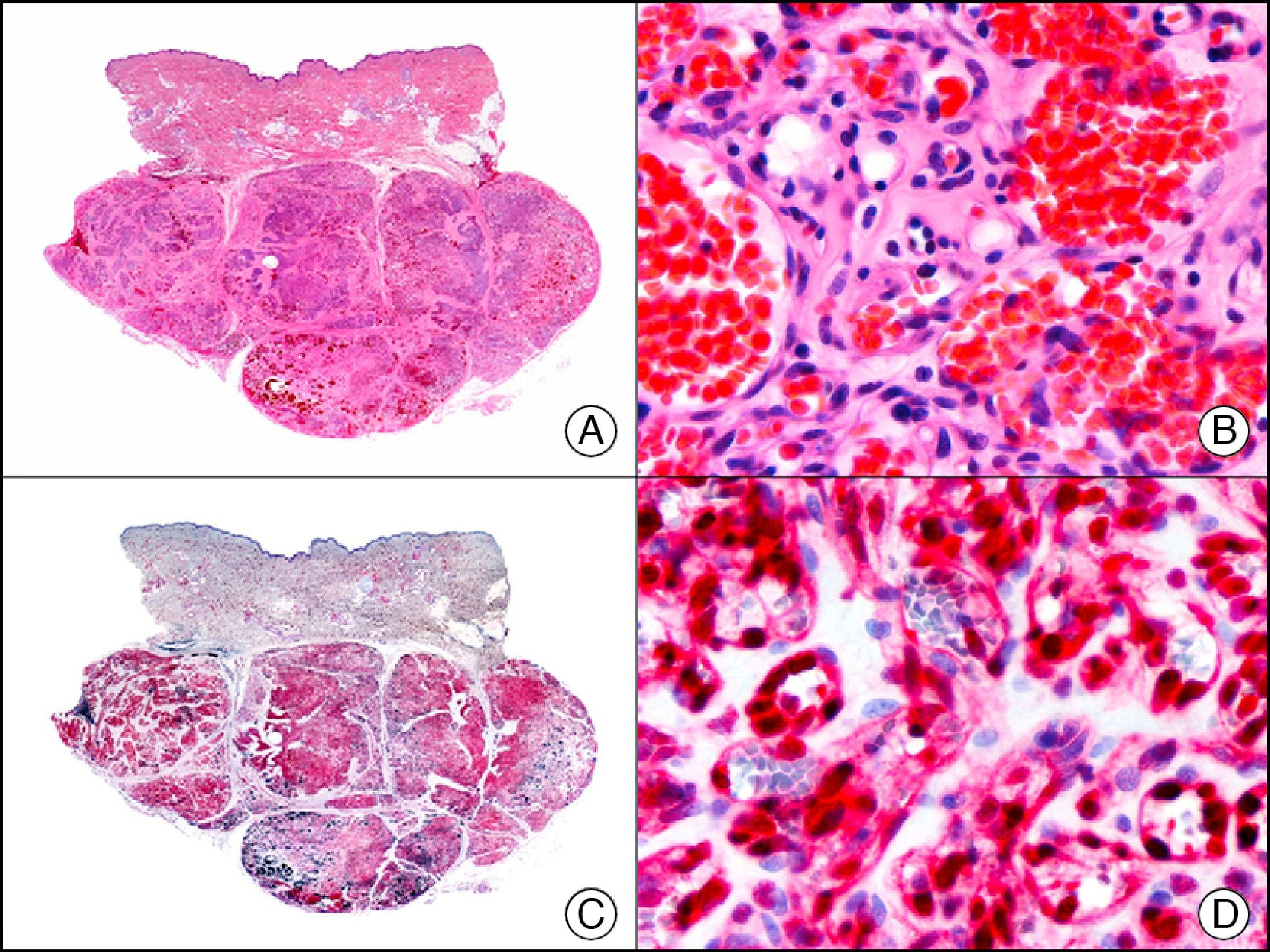
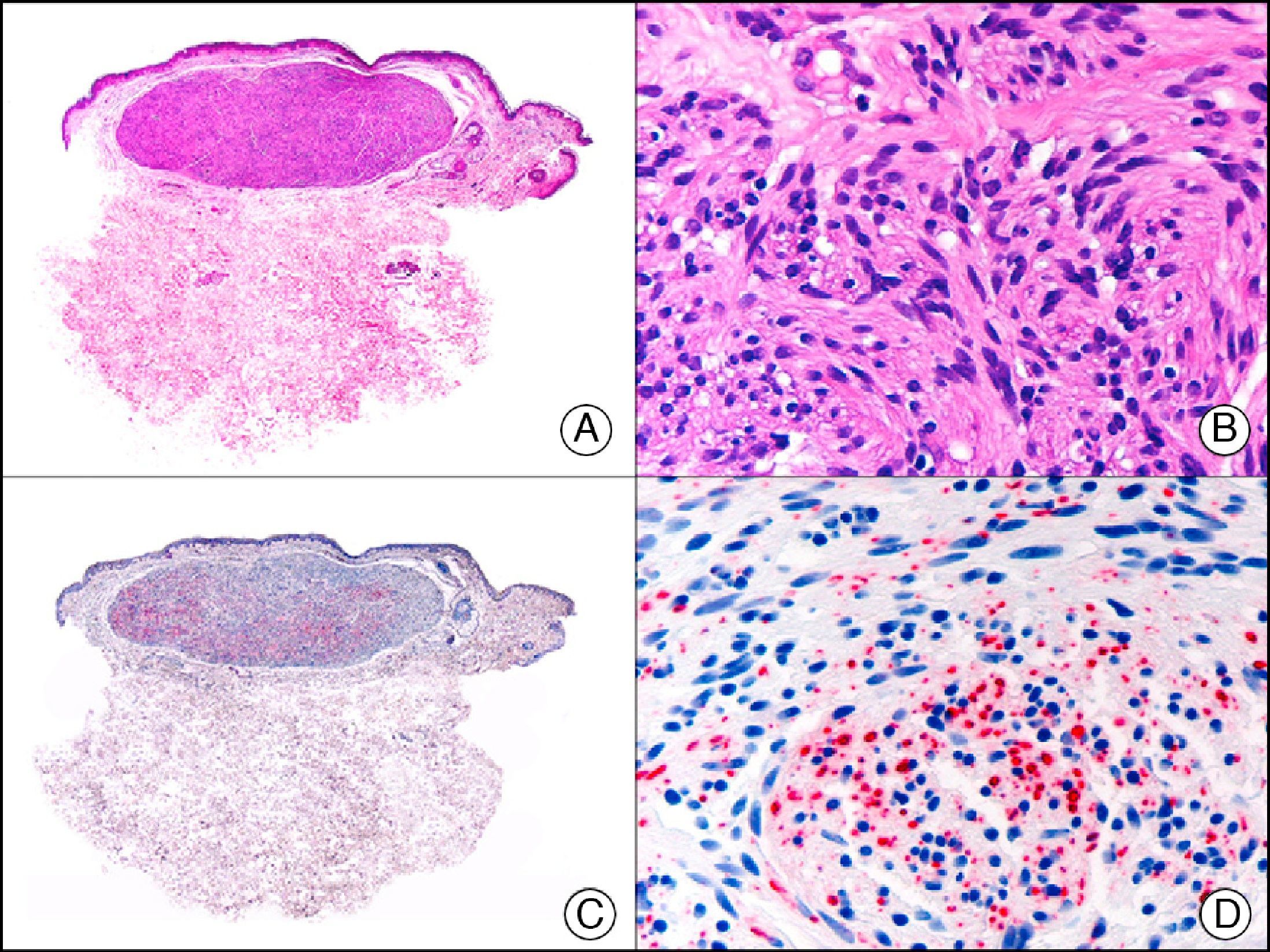
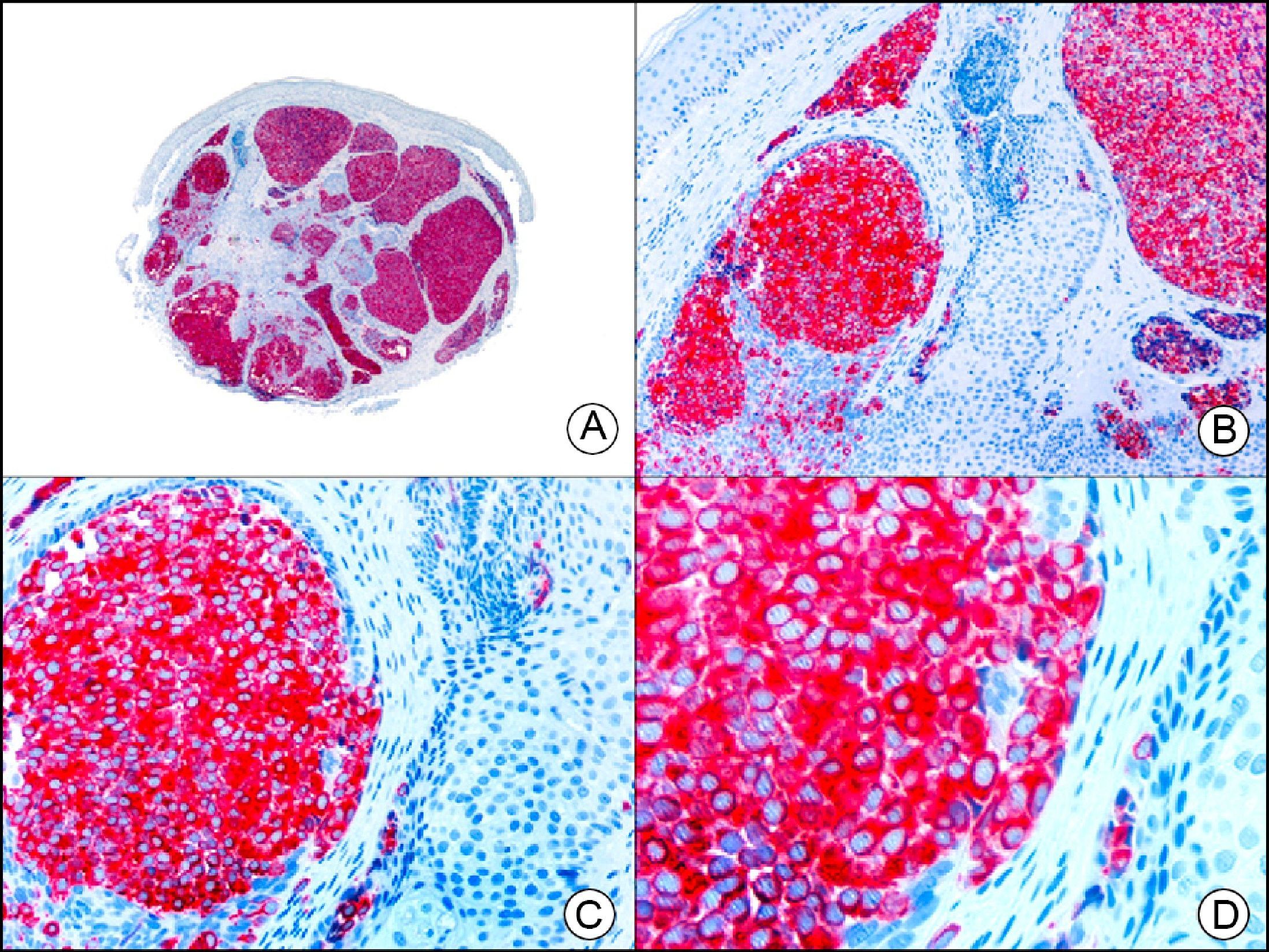
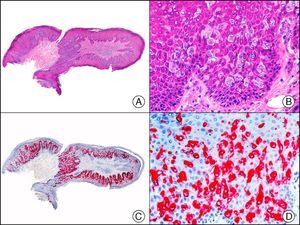
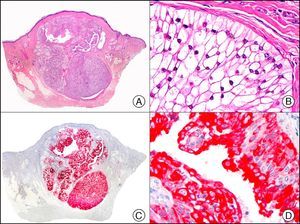
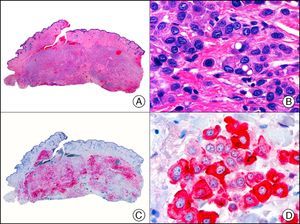
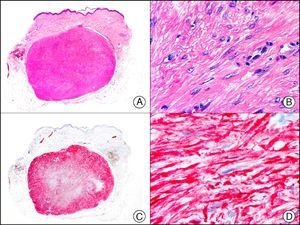
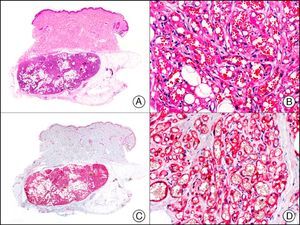
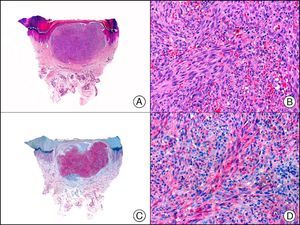
The v-ets erythroblastosis virus E26 oncogene homolog (avian), ERG, is a member of the erythroblast transformation-specific family of transcription factors. It is expressed in normal and neoplastic endothelial cells of blood and lymph vessels and is a highly specific marker of vascular endothelium (Fig. 30). However, it is also positive in neoplastic epithelial cells in 50% of primary and metastatic prostate carcinomas, (although interestingly it is not expressed in normal prostate tissue), as well as in immature myeloid bone marrow cells, chronic myeloid leukemia cells, and Ewing sarcoma cells. Thanks to its high specificity for vascular proliferations, it is very useful in the diagnosis of angiosarcomas, hemangioendotheliomas, and Kaposi sarcoma. Additionally, the fact that it is positive in prostate carcinoma but negative in practically all other carcinomas makes it a very valuable addition to immunohistochemical panels used to investigate cutaneous metastases of unknown origin.58
Lobular capillary hemangioma. A, Low-power magnification (×10). B, Detail of capillary vessels lined with the endothelial cells that constitute the lesion (×400). C, The same sample studied immunohistochemically with ERG (v-ets erythroblastosis virus E26 oncogene homolog [avian]) (×10). D, Detail of ERG-positive nuclei in the proliferating endothelial cells (×400).
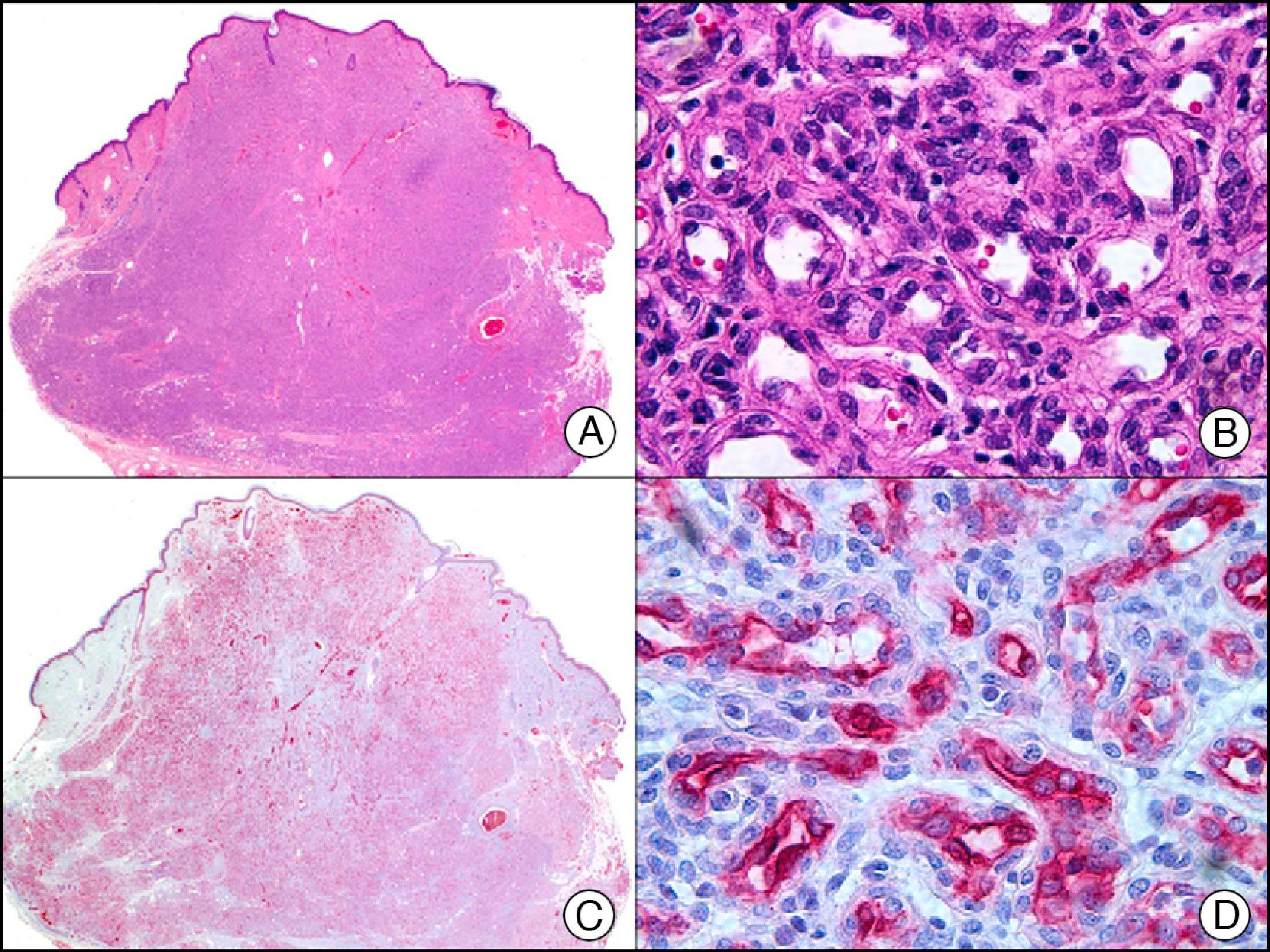
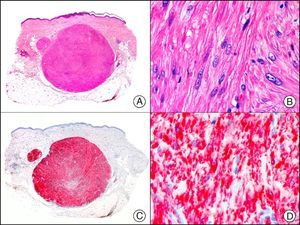
S-100 protein is an acidic protein whose name comes from the fact that it is 100% soluble in ammonium sulfate. It consists of 2 subunits (α and β) with 3 isotypes: a) S-100 αα, expressed in muscle; b) S-100 αβ, expressed in melanocytes, glial cells, chondrocytes, and myoepithelial cells of the secretory coils of eccrine and apocrine glands; and c) S-100 ββ, expressed in Langerhans cells and Schwann cells. S-100 protein is widely distributed throughout the central and peripheral nervous systems and is also present in several nonneural tissues. It is expressed in practically 100% of melanocytic nevi (Fig. 31) and in up to 98% of melanomas, making it the most sensitive marker of melanocytic proliferations. It is, however, also positive in other type of tumors, such as several non-squamous cell carcinomas, myoepithelial tumors, smooth muscle tumors, and peripheral nerve sheath tumors.1
Spitz nevus. A, Low-power magnification (x10). B, Detail of melanocytes with vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (×400). C, The same sample studied immunohistochemically with S-100 protein (×10). D, The neoplastic melanocytes were strongly immunoreactive for S-100 protein (×400).
Melan-A, which is encoded by the MART-1 gene, is a component of the membrane of premelanosomes.1 It is a melanocytic differentiation antigen, recognized by cytotoxic T cells and expressed in normal melanocytes and proliferating cells of melanocytic nevi and melanomas (Fig. 32). It is also expressed in cells of the retina, suprarenal cortex, and ovary and in Leydig testicular cells. Together with S-100 protein, it is the most widely used protein to demonstrate melanocytic differentiation. Melan-A yields a more diffuse but also a more intense staining pattern than S-100 protein. The only melanocytic tumors that are not recognized by Melan-A are desmoplastic melanoma and some spindle cell melanomas.59 Care must be taken when interpreting Melan-A staining patterns in intraepidermal melanocytic proliferations in areas of skin with considerable chronic actinic damage, as the staining of dendritic melanocytes and neighboring keratinocytes can cause a true melanoma in situ to be confused with pigmented actinic keratosis or skin with actinic or chronic sun damage and numerous melanocytes.60 Melan-A is also a very useful tool in the histopathologic evaluation of sentinel lymph nodes in melanoma.
Subungual melanoma in situ. A, Low-power magnification (×10). B, High-power magnification showing numerous melanocytes with hyperchromatic pleomorphic nuclei in the basal and suprabasal layers of the epithelium (×400). C, The same sample studied immunohistochemically with Melan-A (×10). D, Detail of neoplastic melanocytes showing positivity for Melan-A (×400).
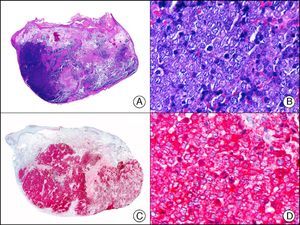
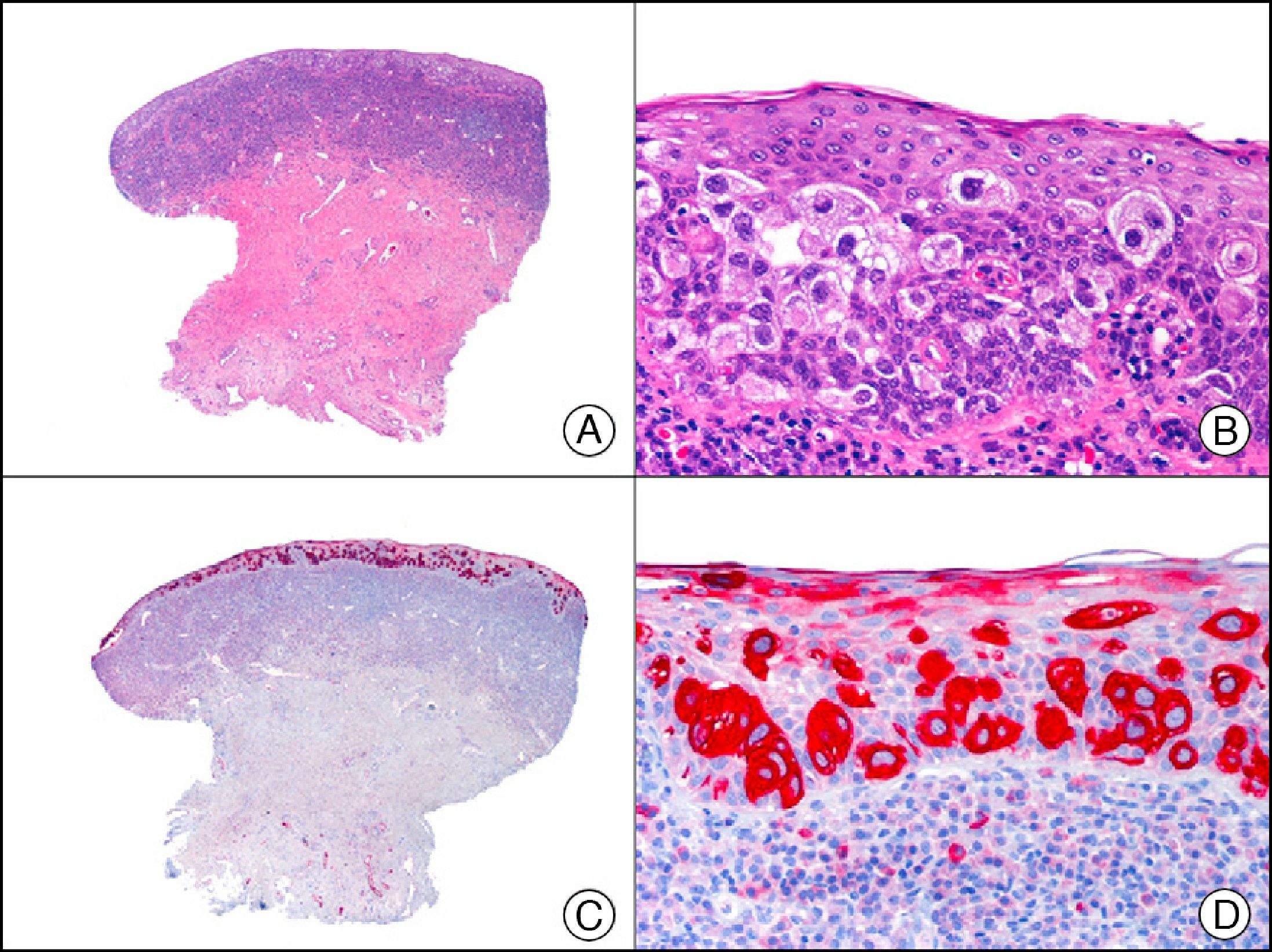
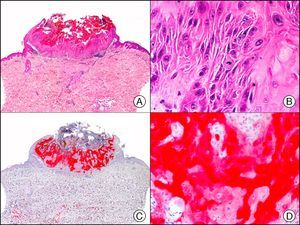
HMB-45 (human melanoma black 45) is a monoclonal antibody directed against a glycoprotein component of premelanosome, gp100. It identifies activated, immature, and intradermal melanocytes, but not adult (quiescent) melanocytes. It has a diagnostic sensitivity of 78% to 93% in melanoma10 (85% for epithelioid melanoma [Fig. 33] and of 30% to 50% for sarcomatoid melanoma), making it one of the most useful markers for confirming a diagnosis of melanoma in cases of malignant tumors of unclear histogenesis. The exception, like with other melanocytic markers, is desmoplastic melanoma, as HMB-45 only labels the intraepidermal and junctional components (it is negative in the dermal component).1,10,61 HMB-45 is perhaps the most sensitive marker of melanocytic proliferations, as it also labels Spitz nevi, blue nevi, and dysplastic nevi.10,59 It also tends to be positive, although only focally, in melanoma metastases.10 Finally, it should not be forgotten that HMB-45 may also be positive in nonmelanocytic lesions containing premelanosomes that have been phagocytized (e.g., melanophages), or transferred to keratinocytes from neighboring dendritic melanocytes, as occurs in pigmented actinic keratosis and other epithelial lesions containing abundant melanic pigment.59
Melanoma in situ. A, Low-power magnification (×10). B, Isolated pagetoid melanocytes scattered through the basal and suprabasal layers of the epidermis (×200). C, The same sample studied immunohistochemically with HMB-45 (human melanoma black 45) (×10). D, Intense staining of neoplastic melanocytes scattered through the upper layers of the epidermis (×200).
MiTF-1 (microphthalmia transcription factor 1) is a nuclear protein involved in the embryologic development of melanocytes and the regulation of melanin synthesis.59 It is expressed in the majority of melanocytic proliferations, although it has low specificity as it is also expressed in macrophages, lymphocytes, fibroblasts, smooth muscle, and Schwann cells. Interpretation is easy given that it is a nuclear marker (Fig. 34). MiFT-1 positivity has been observed in up to 88% of melanoma metastases.10
Blue nevi composed of epithelioid cells. A, Low-power magnification (×10). B, Epithelioid cells with abundant cytoplasm and containing abundant melanin pigment (×400). C, The same sample studied immunohistochemically with MiTF-1 (microphthalmia transcription factor 1) (×10). D, Detail showing positive staining for MiTF-1 in the nuclei of epithelioid melanocytes. Note the absence of staining in the nuclei of melanophages (×400).
SOX-10 (Sry-related HMG-BOX gene 10) is a neural crest transcription factor that appears to play a crucial role in the maturation, maintenance, and differentiation of pluripotent neural crest stem cells towards Schwann cells and melanocytes. It is expressed in all types of benign and malignant melanocytic tumors, but also in neoplastic Schwann cells (e.g., schwannoma, neurofibroma, and malignant nerve sheath tumors) and neoplastic cells of other neuroectodermal tumors (e.g., ganglioneuroma, neuroblastoma, Ewing sarcoma, oligodendroglioma, medulloblastoma, and astrocytoma). SOX-10 is also expressed in myoepithelial cells and their neoplastic proliferations. Like MiTF-1, the fact that it is expressed in the nucleus of cells (Fig. 35) makes results easier to interpret than with S-100 protein, Melan-A, or HMB-45. In addition to having high sensitivity, SOX-10 is a more specific marker of melanocytes than S-100 protein, with studies showing SOX-10 positivity in several desmoplastic melanomas that were negative for S-100 protein.62 It is also useful for distinguishing between the proliferation of dendritic melanocytes sometimes seen in desmoplastic melanoma excision scars and truly persistent desmoplastic melanoma, because SOX-10 has higher specificity for melanoma cells and, unlike S-100 protein, does not recognize dendritic melanomas in scars.63
Desmoplastic melanoma. A, Low-power magnification (×10). B, Detail of neoplastic melanocytes in the reticular dermis (×400). C, The same sample studied immunohistochemically with SOX-10 (Sry-related HMG-BOX gene 10) (×10). D, Detail of SOX-10-positive nuclei in neoplastic melanocytes in the dermis (×400).
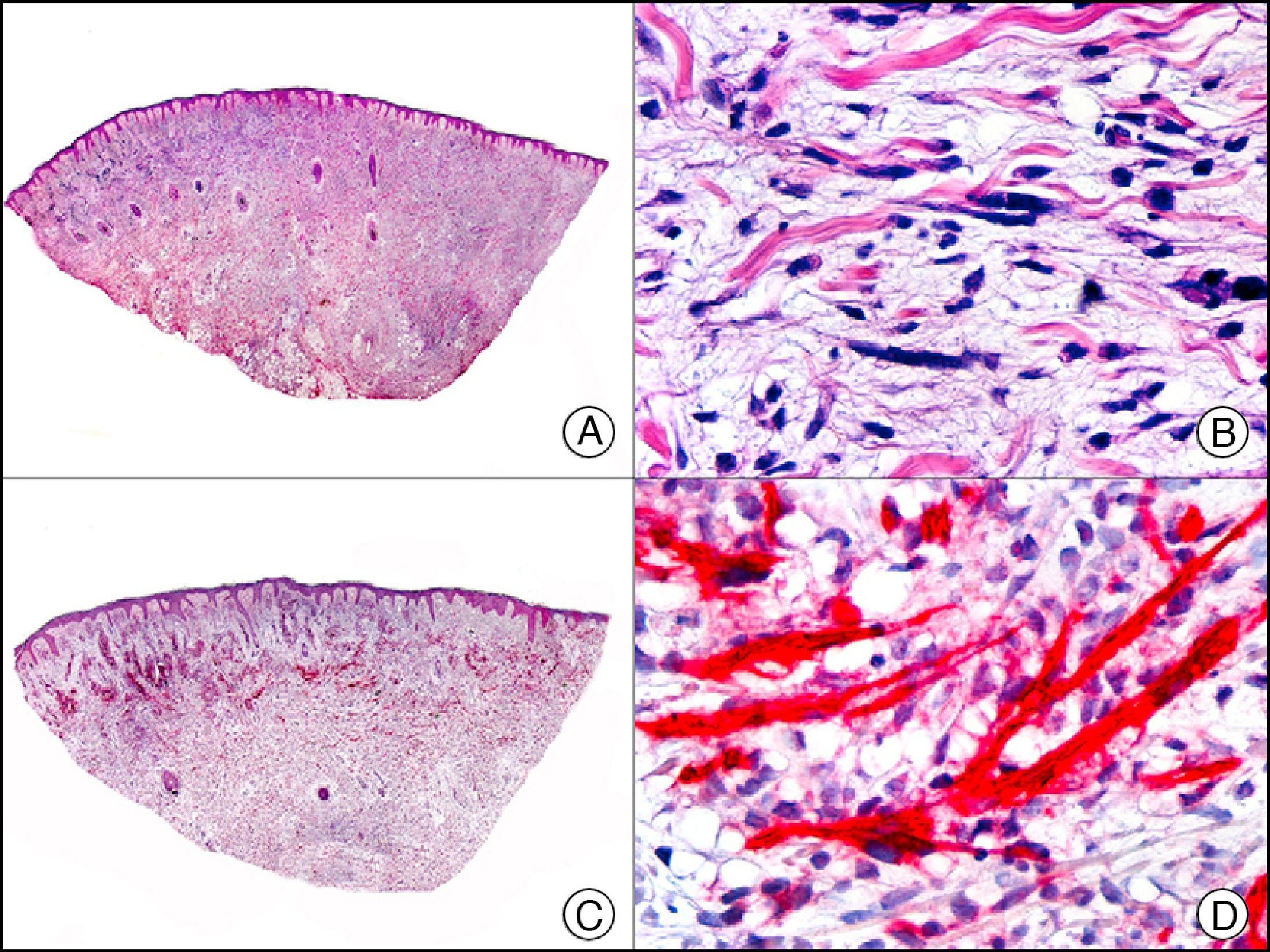
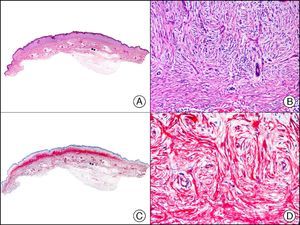
Staining with neurofilament antibodies detects neuronal axons in the peripheral nerves, as well as sympathetic ganglion cells and adrenal medulla cells. It is useful in the diagnosis of neuromas64 (Fig. 36), neurofibromas, ganglioneuromas, neuroblastomas, and pheochromocytomas.
Palisading encapsulated neuroma. A, Low-power magnification (×10). B, Detail of neoplastic cells with spindle-shaped nuclei (×400). C, The same sample studied immunohistochemically with neurofilaments (×10). D, Detail showing staining for neurofilaments in the axons of the proliferating nerve fibers (×400).
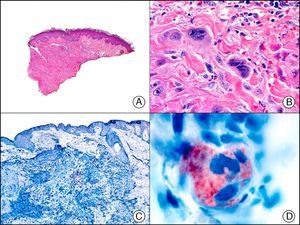
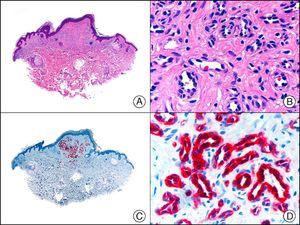
PGP 9.5 (protein gene product 9.5), a protein of unknown function, is present in practically all the cell components of the central and peripheral nerve systems and in many neuroendocrine cells. It is also expressed in the kidney tubule, spermatogonia, and the corpus luteum, and in epidermal Merkel cells and dermal fibroblasts, among others. It is a useful neuronal marker for the investigation of degenerative nerve diseases. In dermatopathology, its presence has been studied in many diseases and positive results have been reported in neoplastic cells of cellular neurothekeoma; indeed, this finding is one of the few arguments supporting the existence of neural differentiation in this enigmatic tumor65 (Fig. 37). PGP 9.5 positivity has also been seen in the cytoplasm of neoplastic cells in certain types of squamous cell carcinoma and keratoacanthoma, with positive results associated with more aggressive behavior in some cases.66
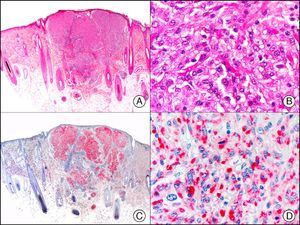
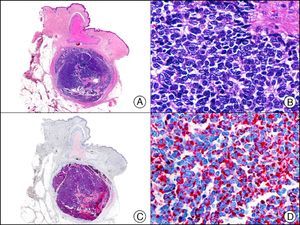
Neuroendocrine Differentiation MarkersEnolase, or phosphopyruvate hydratase, is an enzyme that catalyzes the transformation of 2-phospho-D-glycerate to phosphoenolpyruvate. There are 5 known isoenzymes formed by combinations of 3 subunits (α, β, and γ). Neuron-specific enolase corresponds to the γ isoenzyme, which is formed by 2 γ subunits. It is expressed mainly in neurons, neuroendocrine cells, ganglion cells in the gastrointestinal tract, and nerve fibers. It is positive in glial, neural, and neuroendocrine tumors (small-cell lung carcinoma, Merkel tumor [Fig. 38]), Wilms tumor, and carcinoid tumor), but its low specificity limits its diagnostic value and has led several authors to propose changing its name to nonspecific neuron enolase.
Merkel cell carcinoma. A, Low-power magnification (×10). B, Detail of neoplastic cells showing round nuclei with granular chromatin and numerous mitotic figures (×400). C, The same sample studied immunohistochemically with neuron-specific enolase (NSE) (×10). D, NSE-positive neoplastic cells (×400).
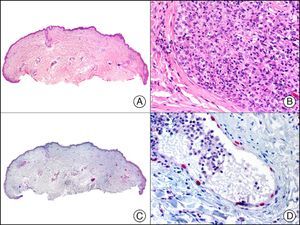
CHR (chromogranin) is a protein of unclear function associated with neurosecretory granules.2 It is expressed in a wide variety of endocrine cells and neurons. Positive staining is specific for neuroendocrine differentiation, but depends on the number of neurosecretory granules, with negative results often seen in poorly differentiated, high-grade tumors. A negative result, therefore, does not rule out neuroendocrine differentiation. In dermatopathology, CHR is used mainly in the study of Merkel cell carcinoma (Fig. 39) and cutaneous metastases from visceral neuroendocrine tumors.
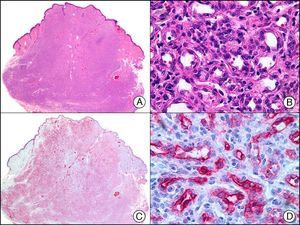
Synaptophysin is a transmembrane glycoprotein expressed in the majority of neuronal and neuroendocrine cells and in their respective proliferations.2 Together with CHR and neuron-specific enolase, it is a common component of immunohistochemical panels used to study primary and metastatic neuroendocrine skin tumors (Fig. 40).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fuertes L, et al. Inmunohistoquímica en dermatopatología: revisión de los anticuerpos utilizados con mayor frecuencia (parte i). Actas Dermosifiliogr. 2013;104:99-127.

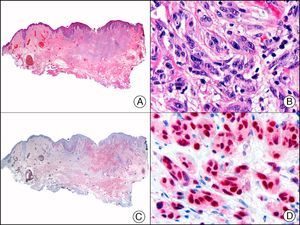
![Angiosarcoma of the breast skin following radiation therapy for breast cancer. A, Low-power magnification (×10). B, Irregular vessels lined with atypical endothelial cells (×400). C, The same sample studied immunohistochemically for amplification of c-Myc (V-myc myelocytomatosis viral oncogene homolog [avian])(×10). D, Most of the nuclei of the neoplastic cells are strongly positive for c-Myc (×400). Angiosarcoma of the breast skin following radiation therapy for breast cancer. A, Low-power magnification (×10). B, Irregular vessels lined with atypical endothelial cells (×400). C, The same sample studied immunohistochemically for amplification of c-Myc (V-myc myelocytomatosis viral oncogene homolog [avian])(×10). D, Most of the nuclei of the neoplastic cells are strongly positive for c-Myc (×400).](https://static.elsevier.es/multimedia/15782190/0000010400000002/v1_201304241618/S1578219013000036/v1_201304241618/en/main.assets/thumbnail/gr29.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
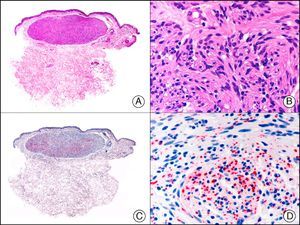
![Lobular capillary hemangioma. A, Low-power magnification (×10). B, Detail of capillary vessels lined with the endothelial cells that constitute the lesion (×400). C, The same sample studied immunohistochemically with ERG (v-ets erythroblastosis virus E26 oncogene homolog [avian]) (×10). D, Detail of ERG-positive nuclei in the proliferating endothelial cells (×400). Lobular capillary hemangioma. A, Low-power magnification (×10). B, Detail of capillary vessels lined with the endothelial cells that constitute the lesion (×400). C, The same sample studied immunohistochemically with ERG (v-ets erythroblastosis virus E26 oncogene homolog [avian]) (×10). D, Detail of ERG-positive nuclei in the proliferating endothelial cells (×400).](https://static.elsevier.es/multimedia/15782190/0000010400000002/v1_201304241618/S1578219013000036/v1_201304241618/en/main.assets/thumbnail/gr30.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)