Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer and its incidence has increased in recent decades. Most cSCCs are successfully treated by surgery, but local and distant metastases develop in approximately 5% of cases; this proportion is higher in certain forms of cSCC with high-risk factors, namely: tumor size >2cm, depth >2mm, Clark level ≥IV, perineural invasion, lymphovascular invasion, poor differentiation, certain histologic subtypes (desmoplastic or adenosquamous carcinoma, invasive Bowen disease, or a cSCC arising in areas of chronic inflammation), immunosuppression, human papillomavirus infection, high-risk anatomic location (pinna of the ear, labial mucosa), expression of certain tumor genes, and inadequate tumor resection. The latest TNM (tumor, lymph node, metastasis) classification of cSCC published by the American Joint Committee on Cancer (AJCC) in the seventh edition of its Cancer Staging Manual now incorporates several of these risk factors to improve disease staging. We review all the factors currently considered to be markers of poor prognosis in cSCC and analyze the new AJCC classification and the different treatment options for high-risk cSCC.
El carcinoma epidermoide cutáneo (CEC) es la segunda neoplasia cutánea más frecuente y su incidencia está aumentando en las últimas décadas. La mayoría de los tumores se van a resolver con cirugía, pero alrededor de un 5% van a presentar metástasis locales y a distancia; esta proporción será mayor en algunos CEC que presenten determinados factores denominados de alto riesgo: tamaño tumoral (mayor de 2cm), profundidad de invasión (superior a 2mm), nivel de Clark (IV o superior), invasión perineural, invasión linfovascular, el grado de diferenciación (tumores pobremente diferenciados), tipo histológico (desmoplásico, adenoescamoso, enfermedad de Bowen invasiva o el CEC que aparece sobre un proceso inflamatorio crónico), inmunosupresión, infección por el virus del papiloma humano (VPH), localización en zonas de alto riesgo (pabellón auricular, mucosa labial), expresión de ciertos genes tumorales, o una inadecuada resección del tumor. La séptima y última clasificación TNM de la American Joint Committe on Cancer (AJCC) ha incluido algunos de estos factores de riesgo obteniendo de esta forma un mejor estadiaje. Realizamos una revisión de todos los factores de mal pronóstico del CEC y analizamos la nueva clasificación de la AJCC, así como las opciones terapéuticas del CEC de alto riesgo.
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer, after basal cell carcinoma (BCC). The incidence of cSCC has increased considerably over the past 20 years, and epidemiological studies predict that it will increase even further in the coming decade.1–4 In Spain, the annual incidence of cSCC per 100 000 population is estimated at 72 for women and 100.8 for men.5 Most cases of cSCC are localized and can be treated by surgical excision or other local procedures.4,6,7 One subset of the disease, however, is more biologically aggressive and has a greater tendency toward local recurrence, lymphatic spread, and, occasionally, invasion of distant organs. The percentage of primary cSCCs that metastasize varies between case series but is usually under 5%. In high-risk cSCC, this percentage is higher,8 ranging from 15%2 to 38%,1 depending on the study. Given the increasing incidence of cSCC and the poor prognosis of a subset of patients, physicians need to be aware of the features that increase the risk of local recurrence and metastases in this type of cancer.
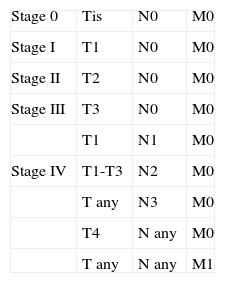
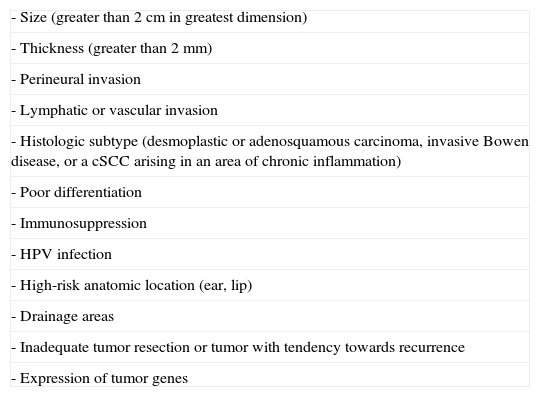
High-risk cSCC has been defined as cSCC with a risk of recurrence, lymph node metastasis, and/or distant metastasis greater than 5%, determined on the basis of tumor characteristics and patient factors.9 The American Joint Committee on Cancer (AJCC) recently published the seventh edition of its Cancer Staging Manual, which contains new TNM staging criteria for nonmelanoma skin carcinomas.6,10 The criteria included in previous editions of the AJCC manual were not appropriate for staging patients with cSCC. The new staging scheme not only considers tumor size (>2cm), but also other important factors, such as thickness (>2mm), Clark level (≥ IV), location (ear, lip), and differentiation (poorly differentiated or undifferentiated).6,10 If a tumor has 2 or more high-risk features, its T classification is upstaged by 1 level, a change that implies a worse prognosis (Tables 1 and 2). Another significant change in this edition of the manual is the fact that cSCC is now staged separately from other nonmelanoma skin carcinomas because it behaves differently from BCC and Merkel cell carcinoma; these separate staging criteria for cSCC were introduced to improve prognostic estimates and approaches to treatment. The new criteria also exclude certain specific sites, such as the eyelid, penis, and vulva. The authors make the point that the majority of cSCC tumors occur on the head and neck.4 cSCC of the eyelid is now staged as an ophthalmic tumor, and penile and vulvar SCC are excluded from the cSCC staging criteria because of their close association with human papillomavirus (HPV) and distinct biological behavior.10 The TNM classification established in the seventh edition of the AJCC manual provides a more accurate prognosis for cSCC; it should be noted, however, that other high-risk features not included in this classification are also associated with higher rates of recurrence and metastasis. Our aim in this article is to review the features of high-risk cSCC and the degree to which they influence prognosis and therapeutic management.
Current TNM Classification According to the Seventh Edition of the Cancer Staging Manual of the American Joint Committee on Cancer (AJCC).
| 1.1 T: Tumor size | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor 2cm or less in greatest dimension with fewer than 2 high-risk featuresa |
| T2 | Tumor greater than 2cm in greatest dimension, or tumor of any size with 2 or more high-risk featuresa |
| T3 | Tumor with invasion of maxilla, mandible, orbit, or temporal bone |
| T4 | Tumor with invasion of skeleton or perineural invasion of skull base |
| 1.2 N: Lymph node involvement | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, 3cm or less in greatest dimension |
| N2 | Metastasis in a single ipsilateral lymph node, more than 3cm but not more than 6cm in greatest dimension; or in multiple ipsilateral lymph nodes, none more than 6cm in greatest dimension; or in bilateral or contralateral lymph nodes, none more than 6cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral lymph node, more than 3cm but not more than 6cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral lymph nodes, none more than 6cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes, none more than 6cm in greatest dimension |
| N3 | Metastasis in a lymph node, more than 6cm in greatest dimension |
| 1.3 M: Distant metastasis | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Larger size, in terms of maximum horizontal diameter, has traditionally been correlated with higher rates of local recurrence and regional metastasis and lower rates of survival. In fact, until the latest edition of the AJCC manual was published, tumor size was the sole criterion used to establish a T classification (Table 1).1 The importance of tumor size has been confirmed by both univariate and multivariate analyses.10 Several studies have established 2cm as the breakpoint after which tumors are more likely to metastasize; tumors larger than 2cm across are twice as likely to recur and 3 times as likely to metastasize as smaller tumors.1,6,10–13 However, physicians should be aware that tumors smaller than 2cm can also metastasize, as observed in a prospective study of 266 patients with metastatic cSCC of the head and neck in which the majority of patients had tumors with a maximum diameter of less than 2cm.9 It is therefore necessary to take other factors into account when determining the risk of metastasis in cSCC.2
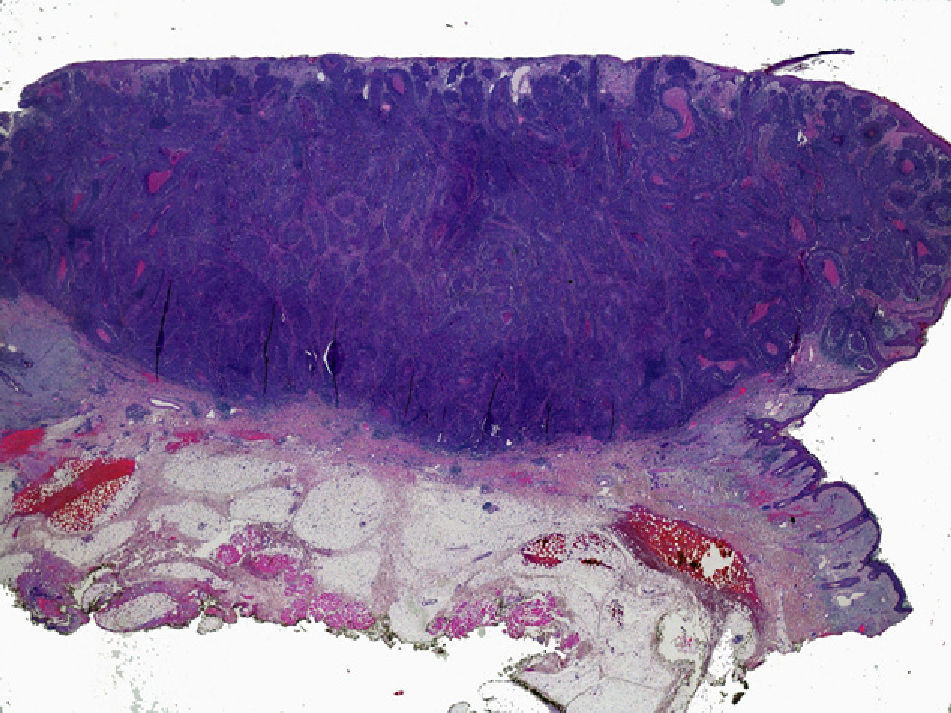
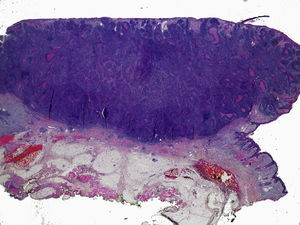
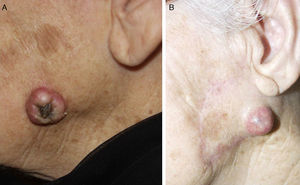
Thickness and DepthTumor thickness is defined as the maximum depth of invasion by the tumor, measured in millimeters; it is equivalent to the Breslow depth in melanoma14 (Fig. 1). The method used to measure the thickness of a cSCC is an important consideration. cSCCs are not easy to measure because they often present as crater-shaped lesions or have a central ulcer. Moreover, measurement criteria vary from 1 pathologist to another. In a 2008 study of 615 patients with cSCC, Branscht et al.11 assessed several variables, including tumor thickness and risk of metastasis, and concluded that cSCCs with a thickness of less than 2mm were associated with practically no risk of recurrence or metastasis. However, the risk of metastasis rose to 4% for tumors 2.1 to 6.0mm thick and to 16% for tumors more than 6mm thick. Veness et al.15 observed no metastases in cSCCs less than 2mm thick. Metastasis did occur, however, in 17% of tumors 2 to 4mm thick and in 83% of tumors more than 4mm thick.
The anatomic depth of invasion, expressed as a Clark level, is an important consideration at sites where the dermis and cell tissue are thinner. For example, a cSCC on the ear is more aggressive than a tumor of the same thickness on the back. Clayman et al.4 observed that cSCCs with a Clark level of IV or higher were associated with a worse prognosis (Fig. 2).
Tumor thickness and depth of invasion are important prognostic factors in cSCC, and both are used to define high-risk features in the latest edition of the AJCC manual (Table 1). The most recent AJCC staging criteria classify invasion of the maxilla, mandible, orbit, or temporal bone as T3 and skull-base invasion as T4 (the latter is associated with a worse prognosis because it implies perineural and bone invasion).10
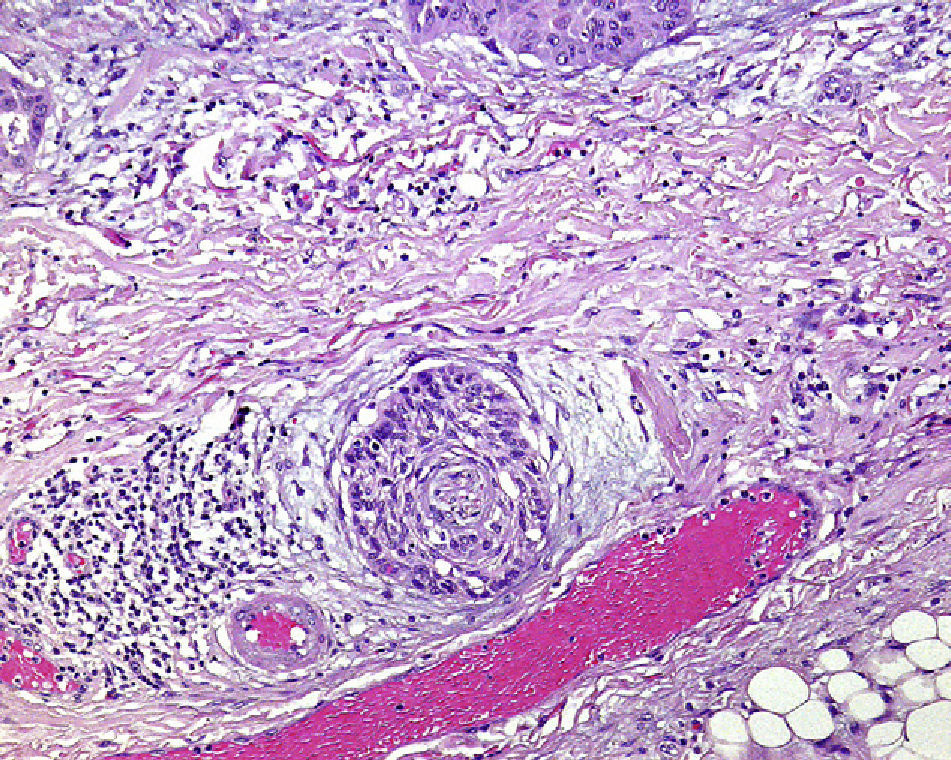
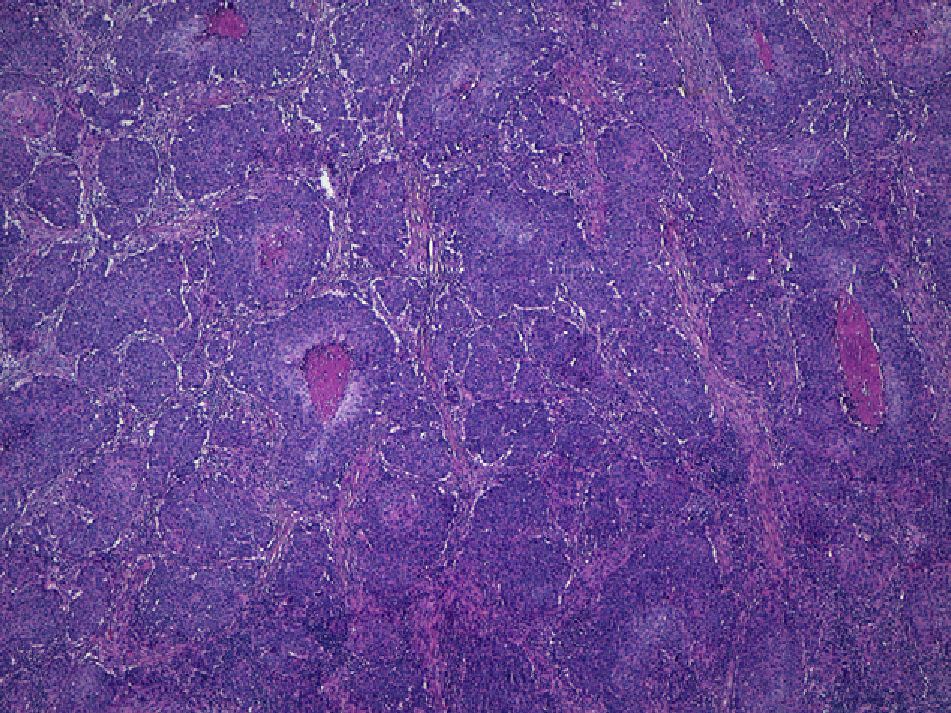
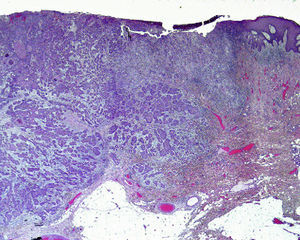
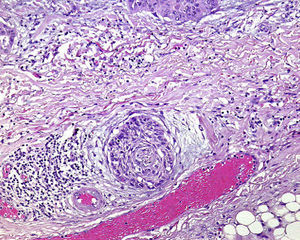
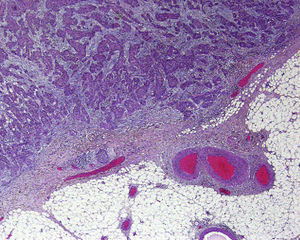
Perineural InvasionPerineural invasion occurs when tumor cells surround and enter a nerve sheath and spread in either direction along the nerve, either towards the surface of the skin or into the deeper tissues16,17 (Fig. 3). This type of spread occurs in 5% to 10% of cases of cSCC. Perineural invasion can be detected clinically and histopathologically and is associated with higher rates of recurrence and lymph node invasion16 as well as lower survival rates4 (Fig. 4). Clinical symptoms of perineural invasion include paresis, paresthesia, pain, and dysesthesia, and histologic evidence of the process can be obtained from a tissue sample following excision. Whether evidenced by clinical or histological manifestations, perineural invasion is always associated with a poorer clinical course owing to the uneven spread of the tumor along the nerves. Perineural invasion in cSCC is associated with poor tumor differentiation, larger size, and higher rates of recurrence. Perineural invasion has consistently been identified as an indicator of poor prognosis10: cSCCs with perineural invasion carry a significantly higher risk of local recurrence, distant metastasis, and disease-specific death than those without such invasion.18 Ross et al.19 recently demonstrated that perineural invasion affecting nerves greater than 0.1mm in diameter is associated with metastasis; they do not consider perineural invasion affecting nerves less than 0.1mm in diameter to be a risk factor, although additional studies are needed to confirm this hypothesis.
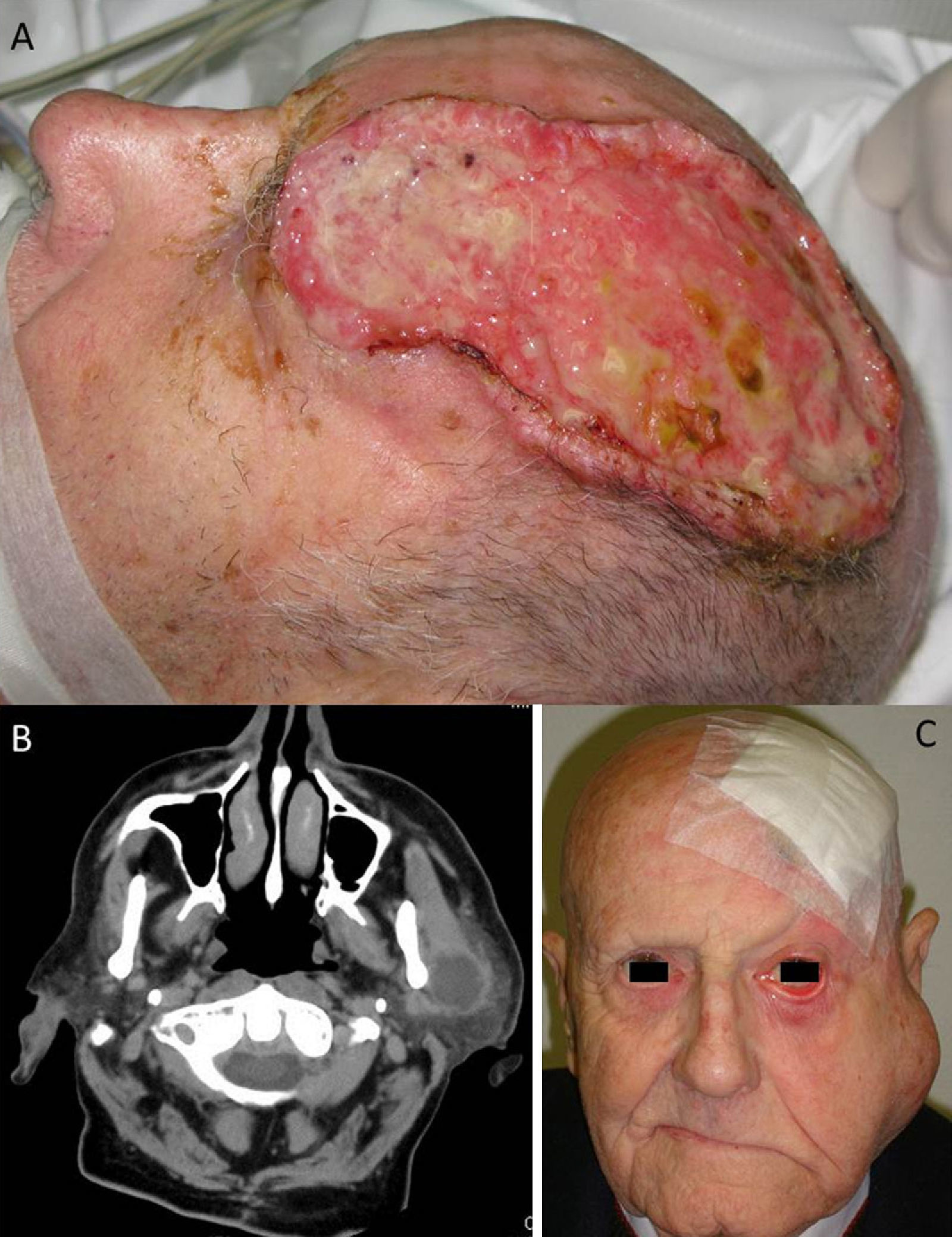
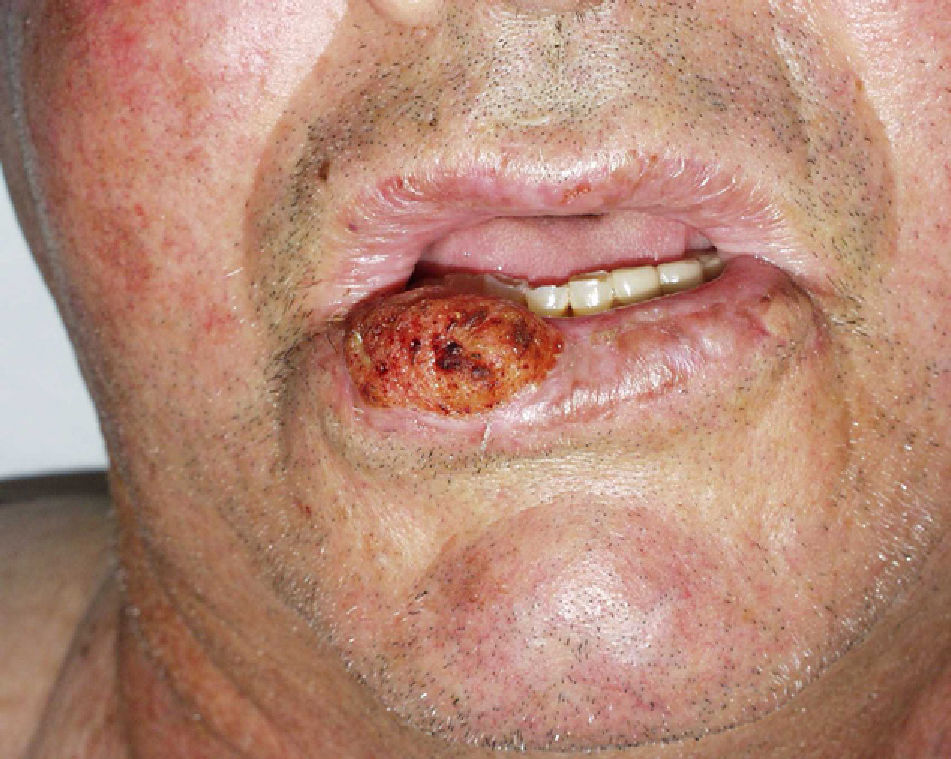
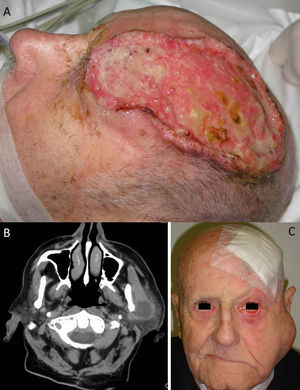
A, Large cutaneous squamous cell carcinoma located in the left frontoparietal region. This tumor has several high-risk features, including its size, its depth, a Clark level of IV (periosteal invasion), and poor differentiation. B, Computed tomography scan of the same patient showing parotid metastases. C, Photograph of the same patient following surgical treatment of the tumor, showing ipsilateral parotid metastases and facial paralysis resulting from tumoral invasion of cranial nerve VII.
Cases of cSCC with lymphatic or vascular vessel invasion have greater risk of regional metastasis. One study found lymphovascular invasion in 40% of patients with lymph node metastases and, by contrast, in 8% of patients without metastatic disease; a multivariate analysis in the same study showed that lymphovascular invasion was a predictor of metastasis.20 Vascular invasion causes cancer to spread via the bloodstream. The most common sites of metastasis are the lungs, liver, bone, brain, and skin.12 Unlike perineural invasion, however, lymphatic and vascular invasion are not considered in most studies, and therefore they are not included in the most recent AJCC staging criteria.10
Histologic SubtypeThere are several histologic subtypes of cSCC, and they do not all exhibit the same behavior. Cassarino et al.21,22 reviewed the histologic subtypes and classified them by metastatic rate. The most aggressive subtypes are desmoplastic carcinoma, adenosquamous carcinoma, and invasive Bowen disease as well as de novo cSCC, defined as a tumor arising not in a precursor lesion such as an actinic keratosis but in a scar or an area of chronic inflammation.22,23
In a subsequent review, Yanofsky et al.24 also identified de novo cSCC and desmoplastic carcinoma as high-risk cSCC variants. The same review also mentioned clear-cell cSCC. Because clear-cell cSCC is very rare, it is difficult to statistically determine its metastatic potential; the authors noted, however, that perineural and perivascular invasion tend to be present in this variant. The review also identified cSCC with single-cell infiltrates as a more aggressive tumor subtype. Other subtypes, in particular cSCC arising in actinic keratosis (the most common variant, accounting for 97% of cases), are more benign. Of these tumors, the most aggressive are those which originate in hypertrophic or proliferative actinic keratoses.22,23
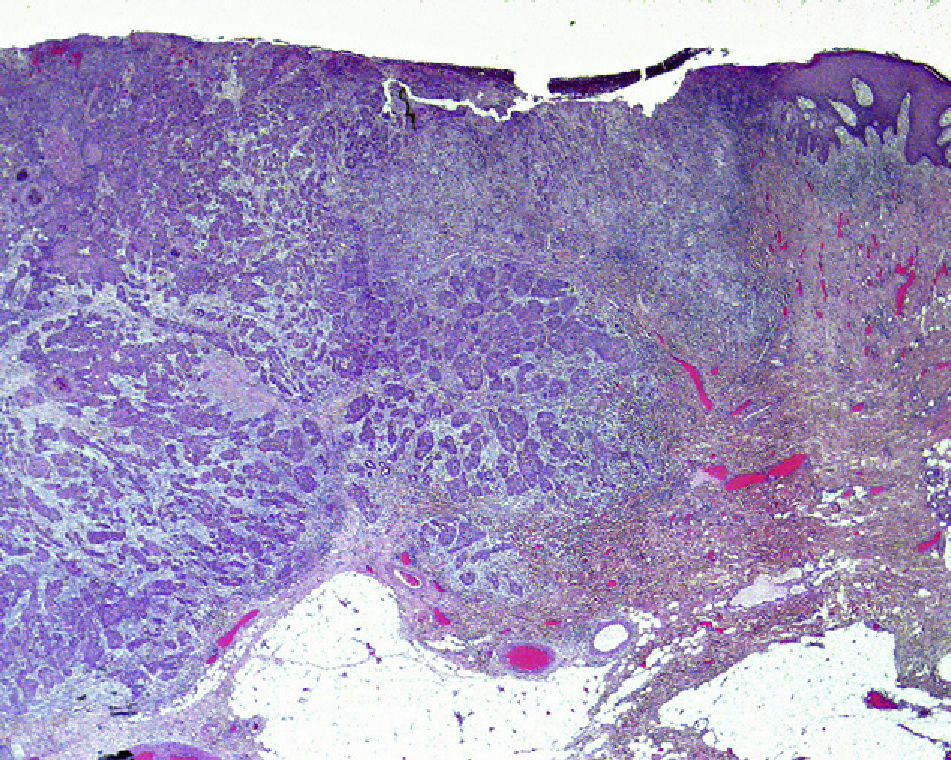
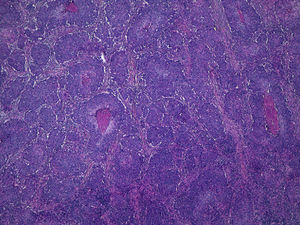
It is also essential to determine the degree of differentiation of a cSCC tumor whatever its histologic subtype. In Broders's index, cSCC tumors are classified by the percentage of differentiated cells: grade I, more than 75%; grade II, 50% to 75%; grade III, 25% to 50%; and grade IV, less than 25%.21 It is common practice to classify cSCCs as well-differentiated, moderately differentiated, or poorly differentiated. Logically, poorly differentiated tumors are the most aggressive.1,6,10,22 The AJCC considers a cSCC tumor to be high grade if it presents poor differentiation, necrosis, deep invasion, high mitotic activity, and spindle cell characteristics. Several studies have shown that poorly differentiated cSCCs have a greater tendency to metastasize4,13 (Fig. 5). Therefore, tumors must be properly classified histologically in order to determine the associated risk.22 Poor differentiation is included as a predictor of poor prognosis in the staging criteria of the seventh edition of the AJCC manual.10
ImmunosuppressionIn immunosuppressed patients, especially recipients of solid-organ (i.e. kidney or heart) transplants, cSCC occurs more frequently and progresses more aggressively than in the general population.15,17 Despite the fact that immunosuppression is not included in the latest AJCC staging criteria, Farasat et al.10 recommend taking it into account by adding an “I” to the staging designation. In the general population, cases of BCC outnumber cases of cSCC by 4 to 1; among recipients of solid-organ transplants, however, cSCC is more common,1 outnumbering cases of BCC by a factor of anywhere from 1.8 to 15, depending on the case series.10,17 One study has shown that patients with both SCC and BCC are less likely to develop further BCCs after initial presentation; this may explain the lower prevalence of BCC among immunosuppressed patients. It should be noted, however, that this study compared patients who had SCC and BCC with patients who only had BCC and did not take immunosuppression into account.25
The likelihood of a patient developing cSCC also depends on the type of transplant he or she has received. cSCC is more likely to occur in heart transplant recipients than in kidney transplant recipients, and less likely to occur in liver transplant recipients than in either of the other 2 groups.9 This difference appears to be related to the degree of immunosuppression used in each type of patient: heart transplant recipients tend to be the most immunosuppressed, followed by kidney transplant recipients and, finally, liver transplant recipients.10,12 Greater immunosuppression may facilitate greater tumor progression. It has recently been postulated, however, that HPV may have an oncogenic role in the development of cSCC in immunosuppressed patients.26,27
In immunosuppressed patients, cSCC is not only more prevalent but also more aggressive and more likely to metastasize. Metastatic rates of approximately 12.9% have been reported in this group of patients.1
The immune deficiency associated with HIV infection is not associated with the development of high-risk cSCCs, except in the case of perianal cSCC, in which HPV plays a crucial oncogenic role. Patients with chronic lymphocytic leukemia tend to develop unusually aggressive and recurrent cSCCs with high-risk features, such as a horizontal diameter of more than 2cm, perineural invasion, and poor differentiation.28
HPV InfectionHPV has a clear causal relationship with mucosal SCC, but no such relationship between HPV and cSCC has been clearly demonstrated. There are various types of HPV. Alpha-HPV infects mucosal sites and is associated with cervical carcinoma and other forms of cancer. Beta-HPV is associated with cSCC.1,29 Studies have shown that more HPV genotypes are found in immunocompromised patients than in immunocompetent patients, although the incidence of HPV infection is also high among the latter. It is estimated that 90% of cSCC tumors in immunocompromised patients contain beta-HPV, compared with 50% in immunocompetent patients.26,29 Infection by a greater number of HPV serotypes has been observed in elderly patients, probably because of immunosenescence, the relative immune deficiency brought on by the aging process.29 Different case series have variously identified HPV 23, HPV 8, and HPV 5 as the most common serotype in cSCC patients,26 although other serotypes have also been associated with the disease.30 Serotypes vary by geographic area.31 The relationship between HPV and more aggressive cSCC has not been proven. Mutations in the EVER1 and EVER2 genes are associated with a higher risk of HPV and a greater likelihood of developing cSCC.31
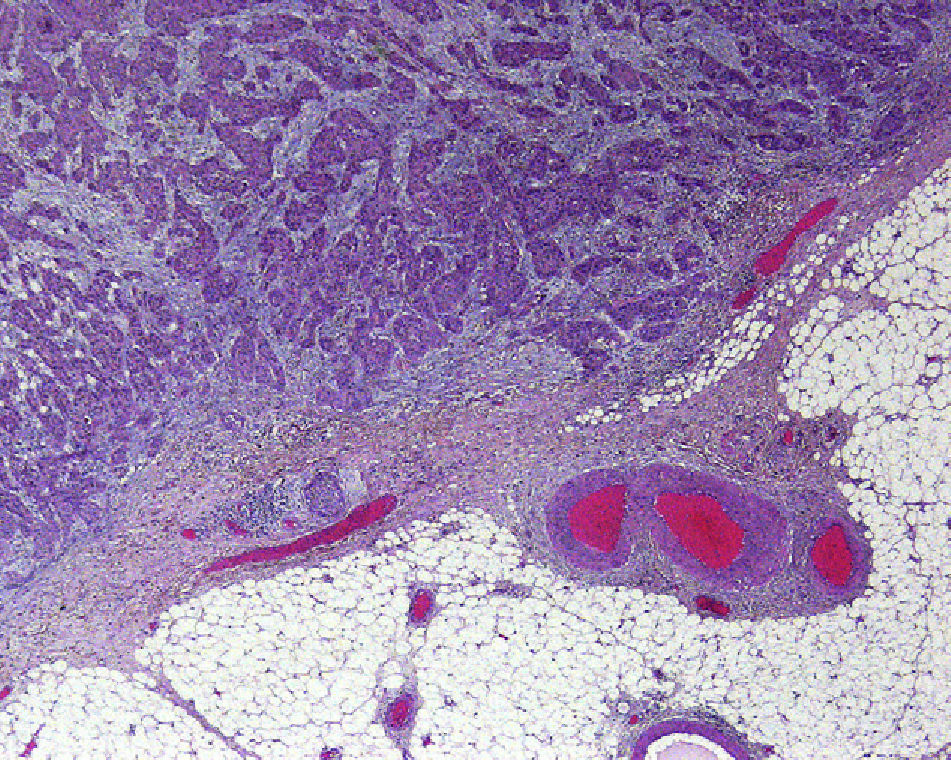
High-Risk Sites and Drainage RegionsThe seventh edition of the AJCC manual specifies 2 high-risk anatomic locations: the ear and the lip.10 Overall, 2% to 6% of cSCCs metastasize, but cSCCs located on the ear and lip have a metastatic rate of 14% to 16%.32 Tumors at these high-risk sites are therefore upstaged.10 This higher metastatic rate has traditionally been attributed to the fact that the ear and lip are highly innervated and vascularized and have little subcutaneous tissue. These attributes facilitate the invasion of deep structures and rapid spread (Fig. 6). In 1 prospective study, however, Brantsch et al.11 observed no difference in the rates of metastasis and recurrence for cSCC tumors of the lip as compared with tumors at other sites; they did, however, find higher metastasis and recurrence rates for cSCCs of the ear. Other authors have identified the scalp as a high-risk location, especially in patients with male pattern baldness, a condition that exposes the scalp to chronic actinic damage.33 cSCC tumors that arise from chronic inflammatory processes—called de novo cSCCs by some authors—are more aggressive.13 De novo cSCCs have been reported to arise from a multitude of chronic processes: burn scars, chronic ulcers, lupus erythematosus, fistulas secondary to chronic osteomyelitis, leprosy, hidradenitis suppurativa, granuloma inguinale, erythema ab igne, congenital poikiloderma, dystrophic epidermolysis bullosa, porokeratosis of Mibelli, necrobiosis lipoidica, lichen sclerosus et atrophicus, lupus vulgaris, and even epidermal cysts.23,34 De novo cSCC has a metastatic rate as high as 38% according to some authors and is often diagnosed late.1 cSCC tumors in areas that drain to the parotid lymph nodes are associated with a worse prognosis; the most frequent site for such tumors is the cheek, followed by the ear, temple, forehead, and scalp35 (Fig. 7). Studies have shown that metastasis to the parotid nodes is associated with poorer disease control and that metastasis to cervical nodes worsens prognosis to a greater extent than isolated parotid lymph node enlargement; a staging system that differentiates between parotid and neck disease has therefore been proposed.32,36 Parotid metastases are predictors of poor outcomes, but the prognosis is worse if the cervical nodes are also involved. In routine practice this staging system has not been adopted and it was not included in the latest AJCC guidelines.10
cSCCs that have been inadequately excised (with very narrow or histologically positive surgical margins) and those that recur despite histologic confirmation of tumor-free margins, are associated with poorer disease control.1,4,17 Up to 50% of tumors with positive margins recur15 and therefore may also metastasize. Although no predefined excision margins have been established for cSCC, this type of tumor requires margins of at least 2 to 4mm (or greater for deeper tumors).17 In a study of patients with metastatic cSCC, Oddonne et al.8 reported that 57% of patients with lymph node metastases had positive or very narrow (less than 2mm) excision margins. In another study, 51% of patients who developed lymph node metastases had a recurrent primary lesion.15 A tumor excised inadequately, with positive or very narrow margins, should therefore be considered to have a high risk of recurrence.
Expression of Tumor Genes and Genetic MarkersVarious histologic and genetic markers are associated with more aggressive cSCCs. The epidermal growth factor receptor (EGFR) is associated with poor prognosis, and tumors that express it are more aggressive.35,37 EGFR is a possible therapeutic target, as inhibition of this pathway has been shown to increase survival. Other proteins, when expressed in cSCC tumors, are also associated with a poor prognosis. STAT3 has been associated with poor differentiation, E-cadherin with lymph node metastasis, and CD44 with recurrent cSCC; in addition, Ets-1 appears to be involved in the pathogenesis of invasive cSCC.1
Management of High-Risk cSCCThe first step in the treatment of high-risk cSCC is to identify the tumor as such according to the features summarized in Table 3. The lack of a prognostic model for cSCC capable of safely and reliably determining an individual's risk of metastasis and death complicates therapeutic decisions and justifies the lack of uniformity in cSCC treatment.28
Summary of High-Risk Features of cSCC.
| - Size (greater than 2cm in greatest dimension) |
| - Thickness (greater than 2mm) |
| - Perineural invasion |
| - Lymphatic or vascular invasion |
| - Histologic subtype (desmoplastic or adenosquamous carcinoma, invasive Bowen disease, or a cSCC arising in an area of chronic inflammation) |
| - Poor differentiation |
| - Immunosuppression |
| - HPV infection |
| - High-risk anatomic location (ear, lip) |
| - Drainage areas |
| - Inadequate tumor resection or tumor with tendency towards recurrence |
| - Expression of tumor genes |
Abbreviations: cSCC, cutaneous squamous cell carcinoma; HPV, human papillomavirus
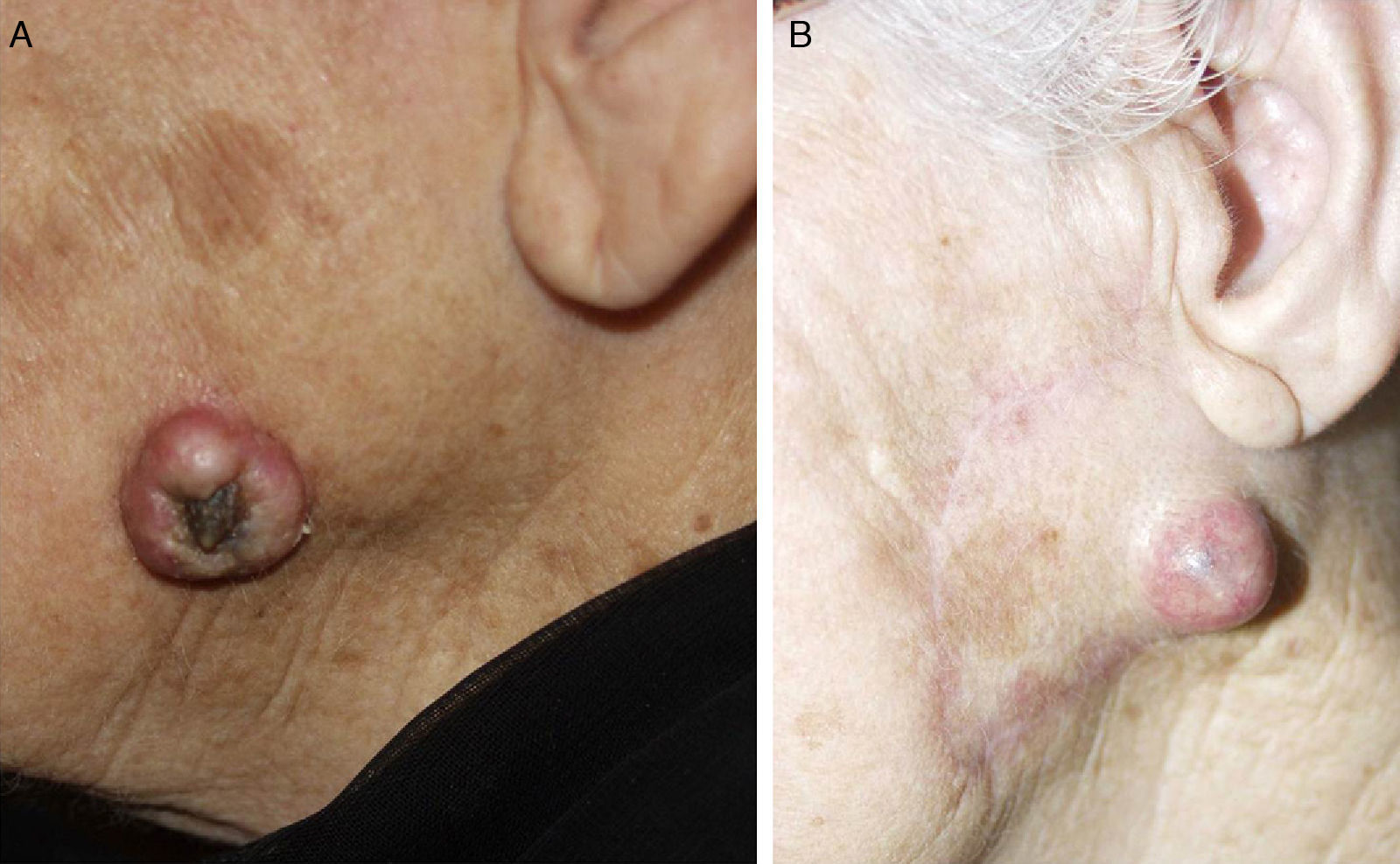
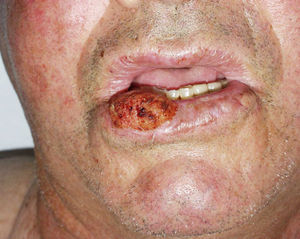
The number of features required to identify a cSCC as high-risk is the subject of some debate. Most authors consider that the presence of just 1 high-risk feature is sufficient to classify a tumor as high-risk, while some authors argue that 2 or more features must be present.15 In any event, most patients with high-risk cSCC exhibit multiple high-risk features (Fig. 8).
Most metastases of cSCC occur within 2 years of the initial diagnosis,1 although there have been reports of late metastasis (5 to 10 years after diagnosis).7,12 Regional lymphatic metastases are not normally detected at the same time as the primary tumor; they are usually found after the primary tumor has been treated.8 In cSCC, 80% of metastases involve locoregional lymph nodes. Distant metastases to solid organs usually affect the lungs, liver, brain, skin, and bones.8
Because cSCCs occur most frequently on the head and neck, regional metastases usually develop in the parotid and/or cervical lymph nodes.4,32 For cSCCs at other sites, affected nodes will depend on the precise location of the tumor. cSCCs on the arms and legs will affect axillary and inguinal lymph nodes, respectively; tumors on the trunk can affect various lymph nodes.
The first-line treatment for high-risk cSCC is Mohs micrographic surgery38 or conventional surgery with margins wide enough to ensure the removal of any malignant infiltration. After surgery, the patient must be followed up regularly to monitor for local and distant recurrences. Patients with high-risk cSCC should undergo routine imaging studies and clinical check-ups every 4 to 6 months. There has been insufficient research to form a consensus on the need for adjuvant radiation therapy, sentinel node biopsy, or prophylactic lymphadenectomy.18,39,40
Tumor StagingBefore any treatment is initiated, a rigorous examination should be performed to rule out regional lymphadenopathy. If any enlarged lymph nodes are found, fine needle aspiration or excisional biopsy should be performed to ascertain whether they are metastatic.28
Radiographic imaging is the standard method for determining the subclinical spread of a tumor; however, the most appropriate imaging technique and the subset of patients who require such studies have not yet been established. Ultrasound of the cervical lymph nodes is an inexpensive, highly sensitive technique recommended by various authors for monitoring cSCC, particularly high-risk cases.11,41 This technique can also be useful for screening and monitoring patients with high-risk cSCC of the head and neck, because the lymph nodes in these areas are usually superficial.28 Computed tomography is generally superior for detecting lymph node central necrosis, extracapsular spread, skull-base invasion, and cartilage involvement.42 Magnetic resonance imaging is better for detecting neurotrophic tumors, defining tissue planes, and distinguishing dense connective tissue from muscle.43 Positron emission tomography is recommended for the detection of metastasis in areas that are necrosed, fibrosed, or affected by radiation therapy. Therefore, this technique is often used following surgery or radiation therapy to detect persistent or recurrent malignancy and to determine its severity.44
Assessment of Immune StatusThe patient's immune status is another important factor in the management of patients with cSCC, especially solid-organ transplant recipients, who are at high risk of developing recurrent metastatic disease.8,13,15,45 Reduction of immunosuppression has been associated with a lower rate of new tumor formation and a better clinical course in aggressive cSCC.28 A reduction of immunosuppression is therefore recommended in immunosuppressed patients who develop cSCC; this should be managed in a multidisciplinary manner by the transplant or oncology team in conjunction with the dermatologist. It should be noted that monotherapy immunosuppression carries less risk than multiple-drug immunosuppression. New immunosuppressants such as sirolimus are associated with a lower incidence of cSCC as compared to calcineurin inhibitors.28
Another factor clearly related to immune status is sun exposure. Because they have fewer repair mechanisms to protect against acute and chronic actinic damage, immunosuppressed patients should always use high-protection sunscreen. Since they have a higher risk of developing skin cancer, immunosuppressed patients should also have regular dermatologic examinations because early diagnosis is the most important step in treating cutaneous tumors.31
Conventional Surgery and Mohs Micrographic SurgeryWhile various approaches have been used to treat high-risk cSCC, complete surgical excision with histologically tumor-free margins remains the best option. Other alternatives, such as cryotherapy, electrocoagulation, topical treatments (imiquimod, 5-fluorouracil, topical retinoids), and photodynamic therapy,1,46 are not recommended for high-risk cSCC.
cSCCs should be appropriately excised with surgical margins of 4 to 10mm, depending on the size of the tumor.1,11 Tumors less than 2cm across are resolved with surgical margins of 4mm in 95% of cases, and surgical margins of at least 6mm are needed to achieve tumor-free margins in tumors with a diameter greater than 2 cm.1 Nevertheless, Mohs micrographic surgery is the preferred treatment for these larger tumors.
Mohs micrographic surgery is the first-line treatment for high-risk cSCC. Conventional surgery has a higher cure rate than Mohs surgery overall, but it is less successful with tumors that are more than 2cm across, poorly differentiated, or recurrent. There have been no studies comparing the effectiveness of Mohs and conventional surgery in the treatment of high-risk cSCC. Mohs surgery is also indicated in cases of cSCC with perineural infiltration. Mohs surgery is not useful in cSCCs with bone invasion, involvement by contiguity of the parotid gland, in-transit metastases, or spread along major nerve branches. In such cases, a multidisciplinary approach is required to ensure complete excision of the tumor.19
Sentinel Node BiopsyThere is no consensus concerning the use of selective sentinel node biopsy (SNB) in patients with high-risk cSCC. SNB makes it possible to avoid unnecessary prophylactic lymphadenectomies and to detect micrometastases. Patients who undergo this procedure can benefit from early treatment, (i.e. lymphadenectomy) before nodal metastasis can be detected clinically or radiologically. SNB is known to be associated with much lower morbidity than lymphadenectomy, but there have been no prospective studies comparing SNB and prophylactic lymphadenectomy. Such studies would be necessary in order to determine whether SNB increases survival or not.47 Several authors have recommend the use of SNB in immunosuppressed patients and in cSCCs that are recurrent, poorly differentiated, larger than 4cm across, or which arise in a chronic inflammatory lesion, a scar, the lip, or the ear.40 However, other authors recommend the use of SNB on any patient with a cSCC that exhibits a high-risk feature.35
Prophylactic LymphadenectomyProphylactic lymphadenectomy is the removal of the ipsilateral neck lymph nodes when a tumor is at stage N0, defined either clinically or with imaging techniques. Some authors recommend performing a prophylactic lymphadenectomy when any high-risk features are present.1 Veness17 reported that up to 24% of patients with no clinical evidence of nodal involvement who underwent a prophylactic lymphadenectomy did, in fact, have local metastases. However, the decision to perform a prophylactic lymphadenectomy solely on the basis of high-risk features is controversial because prophylactic lymphadenectomy is an aggressive procedure associated with high morbidity, and because high-risk cSCC usually occurs in elderly patients with comorbidities,33 a factor that further limits the number of cases in which the procedure is indicated.
Radiation TherapyRadiation therapy can be used to treat high-risk cSCC, but it generally yields poorer outcomes than surgery. The use of radiation therapy is limited by the fact that the technique does not allow the confirmation of margins, an especially important concern in the case of large or thick tumors. Radiation therapy is therefore generally reserved for elderly patients with inoperable tumors. However, at some sites, such as the lower lip, radiation therapy achieves results similar to those obtained by surgical treatment and can provide better functional and cosmetic outcomes.17
Adjuvant Radiation TherapyAdjuvant radiation therapy has been used in some patients with high-risk SCCs, especially in cases where perineural invasion, positive (or indeterminate) surgical margins, or in-transit metastases are present. There have been no randomized studies comparing the outcomes of surgery plus adjuvant radiation therapy to the outcomes of surgery alone.18 The benefits of adjuvant radiation therapy are therefore subject to debate, although many authors have recommended this technique.17,18,35
cSCCs with significant perineural invasion (i.e. affecting nerves with a diameter greater than 0.1mm) have higher rates of recurrence, even following excision with tumor-free surgical margins. Adjuvant radiation therapy is indicated in such cases, although the utility of this approach has not been clearly established.19 In cases of cSCC with perineural invasion in which complete surgical excision is not possible, radiation therapy is used despite poor prognosis.39 Adjuvant radiation therapy has also been used to treat cSCCs with positive or indeterminate surgical margins, but the risk of local recurrence and regional or distance metastasis is higher than in cases where the margins are free of neoplastic infiltration.28
ChemotherapyWe now have chemotherapy drugs that have been shown to increase survival in patients with high-risk or locally advanced cSCCs. Small doses of oral retinoids reduce the number of cSCCs and recurrence in immunosuppressed patients and are therefore indicated for patients with multiple high-risk tumors.45,46 Oral capecitabine or its active form, oral 5-fluorouracil, have been shown to effectively treat locally advanced cSCCs.1 Good results have also been obtained by combining oral 5-fluorouracil with subcutaneous interferon alfa for 2 to 3 weeks, and by combining oral retinoids with subcutaneous interferon alfa.28,46 Drugs that inhibit EGFR, a receptor expressed in aggressive cSCCs, have also shown good preliminary results.46 This category of drugs includes gefitinib, which in some trials has been shown to increase survival in patients with cSCC. EGFR inhibitors are used to treat other types of cancer, such as lung and colorectal carcinomas, and have been shown to increase survival. Cetuximab, another EGFR inhibitor, has yielded positive results both alone and in combination with 5-fluorouracil and cisplatin, and has been shown to improve prognosis in patients with locally advanced cSCC, both with and without radiation therapy.46,48–50
ConclusionsThere is no well-established definition of high-risk cSCC. Nevertheless, it is usually defined as cSCCs associated with a greater than 5% risk of recurrence, lymph node metastasis, and/or distant metastasis (risk level being determined on the basis of certain high-risk features). In the seventh and most recent edition of its Cancer Staging Manual, the AJCC updated its staging criteria to include features that are predictors of a poorer clinical course. The result is a better staging scheme that provides a more accurate prognosis for patients. However, other high-risk features also associated with poorer outcomes were not included in the revised staging criteria. The following are the features associated with high-risk cSCC: tumor size greater than 2cm, thickness greater than 2mm, a Clark level of IV or higher, perineural invasion, lymphatic or vascular invasion, poor differentiation, certain histologic subtypes (desmoplastic or adenosquamous carcinoma, invasive Bowen disease, or cSCCs arising in areas of chronic inflammation), immunosuppression, HPV infection, high-risk anatomic location (ear or lip), expression of certain tumor genes, and inadequate tumor resection.
In the therapeutic management of high-risk cSCC, it is essential to detect any high-risk features, stage the disease properly, assess the patient's degree of immunosuppression, and treat the tumor surgically, either with conventional surgery with sufficient margins or, preferably, with Mohs surgery. In certain cases, SNB or lymphadenectomy should be considered, although there is no consensus regarding whether SNB increases survival. Various authors have recommend the use of SNB in immunosuppressed patients and patients with cSCCs that are poorly differentiated, more than 4cm across, recurrent, or have arisen in a chronic inflammatory lesion, a scar, the lip, or the ear. However, other authors recommend the use of SNB on any patient with a cSCC that exhibits any high-risk feature. Radiation therapy or adjuvant radiation therapy may also be useful in certain cases, and chemotherapy, used in some cases of cSCC with distant metastasis, has shown good results.
Therefore, it is very important to identify patients with high-risk cSCC, monitor them closely, detect recurrence and metastases early, and provide more aggressive treatment in order to reduce the morbidity and mortality associated with this disease.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Nuño-González A, Vicente-Martín FJ, Pinedo-Moraleda F y López-Estebaranz JL. Carcinoma epidermoide cutáneo de alto riesgo. Actas Dermosifiliogr.2012;103:567-578.