The mitogen-activated protein kinase (MAPK) pathway is the therapeutic target in a large number of neoplasias. MEK inhibitors such as trametinib have been used to treat solid tumors and blood cancers in adults, whereas its use in children is extremely rare.1 Cutaneous manifestations associated with these therapies are frequent, but little data exists on hair abnormalities. We present the case of a girl who developed trichoschisis and trichorrhexis nodosa during treatment with trametinib.
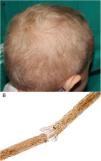
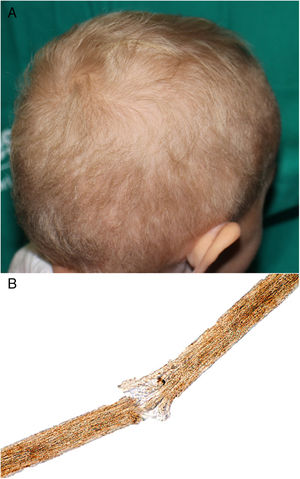
A 2-year-old Caucasian girl with a history of neurofibromatosis type 1 (NF1), which had been diagnosed at the age of 9 months, and carrier of a de novo heterozygous mutation in c.7006G>T presented optic glioma and cervical plexiform neurofibroma with involvement of the nerve roots and occupation of the spinal canal, vascular compression and a risk of involvement of the airways. Treatment was instated with trametinib, for compassionate reasons, at the age of 14 months. Ten months later, she was sent to our department due to the appearance of papules and pustules grouped in the dorsal area, some of which were umbilicated and clinically suggestive of molluscum contagiosum; the lesions had appeared several days earlier. The patient also presented erythematous papules on the tip of the nose and in the perioral region, angular cheilitis, and fine blond hair with a dull appearance; these manifestations appeared 2 months after beginning treatment (Fig. 1A). The patient had no hypertrichosis or trichomegaly and presented no other abnormalities on the skin or nails. She did not use chemical products or hairstyles that might cause traction. Study of the hair under an optical microscope revealed trichorrhexis nodosa and multiple trichoschisis fractures (Fig. 1B). Gram staining revealed gram-positive cocci on the surface of the skin lesions, PAS staining revealed no fungi, and immune staining for herpes virus was negative. The lesions improved after application of topical fusidic acid. As the change in the hair was only an esthetic alteration, it was decided, on consultation with the mother, not to indicate treatment. The patient was transferred to the city and long-term follow-up has therefore not been possible.
New targeted therapies have been developed in recent years to treat some solid tumors and hematologic cancers. Cutaneous manifestations are frequent in patients treated with MEK inhibitors.2,3 Few cases of these reactions are recorded in adults and even fewer in children, for whom MEK inhibitors are not currently approved and are only indicated on compassionate grounds.1 The most commonly described cutaneous alterations include acneiform eruptions, angular cheilitis, xerosis, folliculitis, and finer hair, as in our patient, as well as seborrheic dermatitis and paronychia1–6 (Table 1).
Cutaneous Manifestations of Pediatric Patients Treated with Trametinib.
| Patient | Age, y | Sex | Neurofibromatosis | Tumor | Acneiform Eruption | Angular Cheilitis | Xerosis | Seborrheic Dermatitis | Folliculitis | Paronychia | Fine Hair |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daysa | Weeksa | Weeksa | Daysa | Weeksa | Weeksa | ||||||
| 1 | 3 | F | Astrocytoma | 4 | 12 | ||||||
| 2 | 9 | F | Optic glioma | 23 | 8 | ||||||
| 3 | 10 | M | X | Optic glioma | 2 | 2 | 12 | ||||
| 4 | 13 | F | Optic glioma | 10 | 10 | 28 | 2 | ||||
| 5 | 13 | F | X | Malignant tumor of peripheral nerve sheath | 10 | 2 | 2 | ||||
| 6 | 13 | F | X | Plexiform neurofibroma | 1.5 | 12 | X | ||||
| 7 | 15 | F | X | Optic glioma | 15 | 1.5 | 11 | X | |||
| 8 | 17 | M | X | Astrocytoma | 11 | 16 | 2 | 14 | 1 | X | |
| 9 (this case) | 2 | F | X | Plexiform neurofibroma and optic glioma | 38 | 40 | 40 | X |
Abbreviations: F indicates female; M, male.
Time from start of treatment to appearance of clinical signs.
Source: modified from Boull C et al.1
Pilose dysplasias are malformations of the hair stem or bulb and may form part of a genetic syndrome or be nonspecific. Trichorrhexis nodosa is the most common pilose dysplasia and corresponds to a structural defect of the hair, characterized by transversal fractures in the hair stem, which takes on the appearance of a brush at each side of the break. Clinically, it manifests as white or yellowish particles joined to the hair stem. It usually affects head hair, but may appear on the eyebrows, eyelashes, or body hair. It occurs due to mechanical or chemical trauma in previously normal or abnormally fragile hair. Examples of physical trauma includes excessive brushing, hairstyles with traction, exposure to heat or ultraviolet light, trichotillomania, and scratching. Chemical trauma includes frequent washing, use of products for permanent waves, dyes, and exposure to salt water.7 Congenital (proximal) trichorrhexis nodosa has also been linked to hypothyroidism, citrullinemia, argininosuccinic aciduria, Menkes syndrome, trichothiodystrophy, tricho-hepato-enteric syndrome, and one reported case in a patient treated with anti-TNF-α.8,9 Trichoschisis is s transverse fracture of the hair that, although it has been linked to trichothiodystrophy, is not a specific finding and may be present in normal hair.
Treatment of these hair abnormalities is difficult and includes cutting off the damaged hair, using conditioners, and avoiding trauma. Treatment of the possible underlying causes must also be considered. Specific diets for specific deficits, use of minoxidil 5%, and sodium levothyroxine have been described, but only in isolated cases.7,10
In conclusion, we present the case of a pediatric patient with NF1 who presented trichoschisis and trichorrhexis nodosa during treatment with trametinib. Although abnormalities of the hair follicle associated with some targeted therapies are common, we have found no other cases in which the histologic characteristics of the hair, or the association of trichoschisis or trichorrhexis nodosa have been described in patients treated with trametinib, and we have found only 1 case describing a patient with uncombable hair and NF1.11
Determining the chronology of the appearance of the manifestations associated with these therapies is useful for predicting them and instating appropriate treatment, thereby avoiding having to reduce the dosage of or suspend the MEK inhibitors unnecessarily.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Giacaman A, Quintero A, Salinas Sanz JA, Martín-Santiago A. Cambios en el pelo asociados a trametinib. Actas Dermosifiliogr. 2020;111:441–444.