Superficial mycoses are some of the most common diseases worldwide. The usual culprits—yeasts belonging to the genera Malassezia and Candida—are commensal species in the skin that can cause opportunistic infections. We aimed to determine whether these yeasts use glycosaminoglycans (GAGs) as adhesion receptors to mediate binding to epithelial cells.
Material and methodsIn keratinocyte and dermal fibroblast cultures, we used rhodamine B and genistein to inhibit GAG synthesis to study the role these molecules play in the adhesion of Candida albicans and Malassezia species to cells. We also analyzed GAG involvement by means of enzyme digestion, using specific lyases.
ResultsRhodamine B partially inhibited the adhesion of both fungi to keratinocytes but not to fibroblasts. Selective digestion of heparan sulfate enhanced the binding of Malassezia species to keratinocytes and of both fungi to fibroblasts. Chondroitin sulfate digestion decreased Calbicans adhesion to keratinocytes, but increased the adhesion of the filamentous forms of this species to fibroblasts.
ConclusionsCell surface GAGs appear to play a role in the adhesion of Calbicans and Malasezzia species to keratinocytes. In contrast, their adhesion to fibroblasts appears to be enhanced by GAG inhibition, suggesting that some other type of receptor is the mediator.
Las micosis superficiales son algunas de las enfermedades más comunes en todo el mundo, siendo los agentes causales más frecuentes las levaduras de los géneros Malassezia y Candida, comensales habituales de la piel que pueden actuar como patógenos oportunistas. El objetivo de este trabajo es investigar si los glicosaminoglicanos (GAG) de las células epiteliales son utilizados por estos microrganismos como receptores de adhesión a las mismas.
Materiales y métodosSe utilizaron cultivos de queratinocitos y fibroblastos dérmicos. La participación de los GAG en la adhesión de Candida albicans (C. albicans) y Malassezia spp. se estudió mediante inhibición específica de la síntesis de estas moléculas empleando rodamina B o genisteína. También se analizó mediante digestión enzimática in situ empleando liasas específicas.
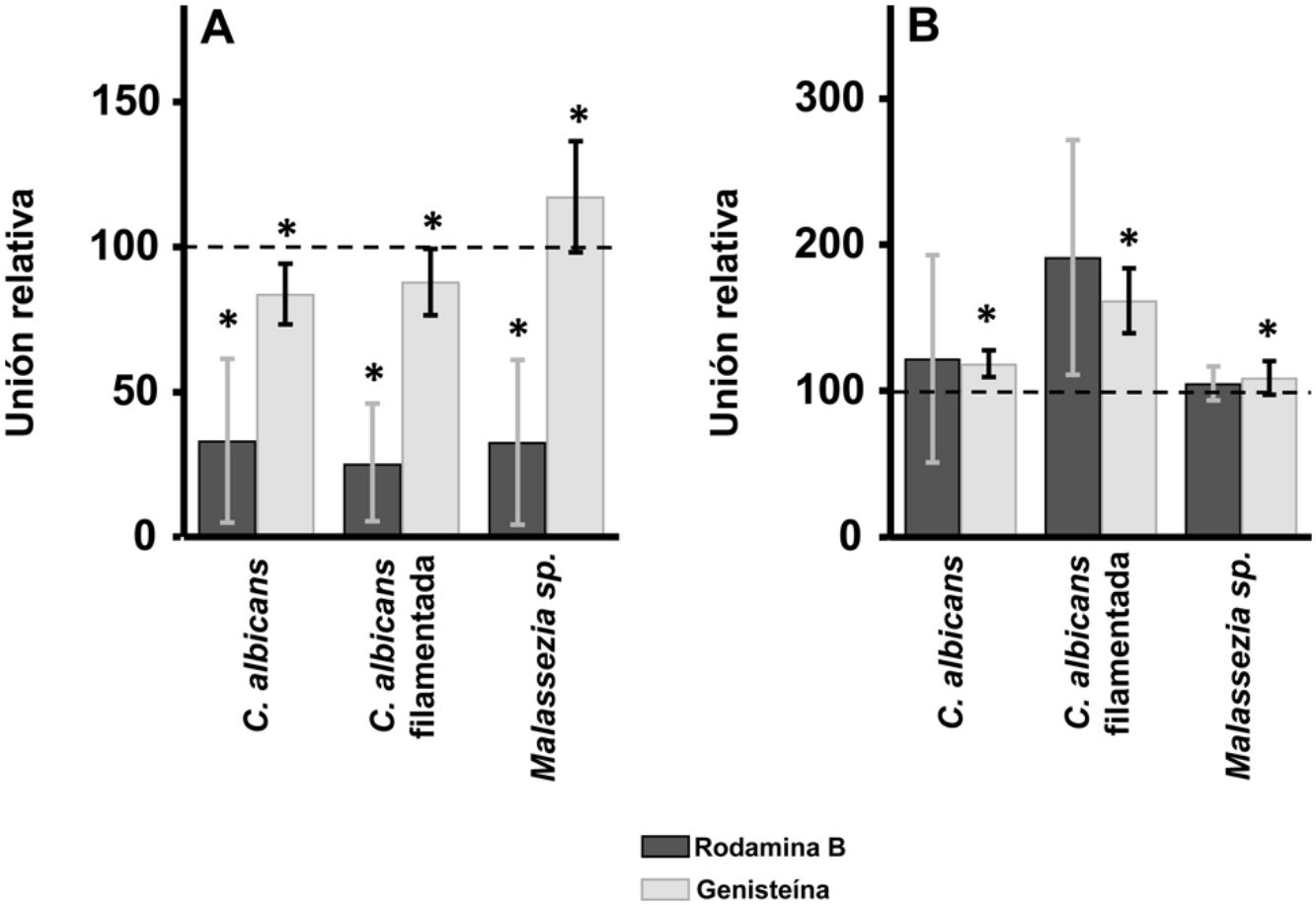
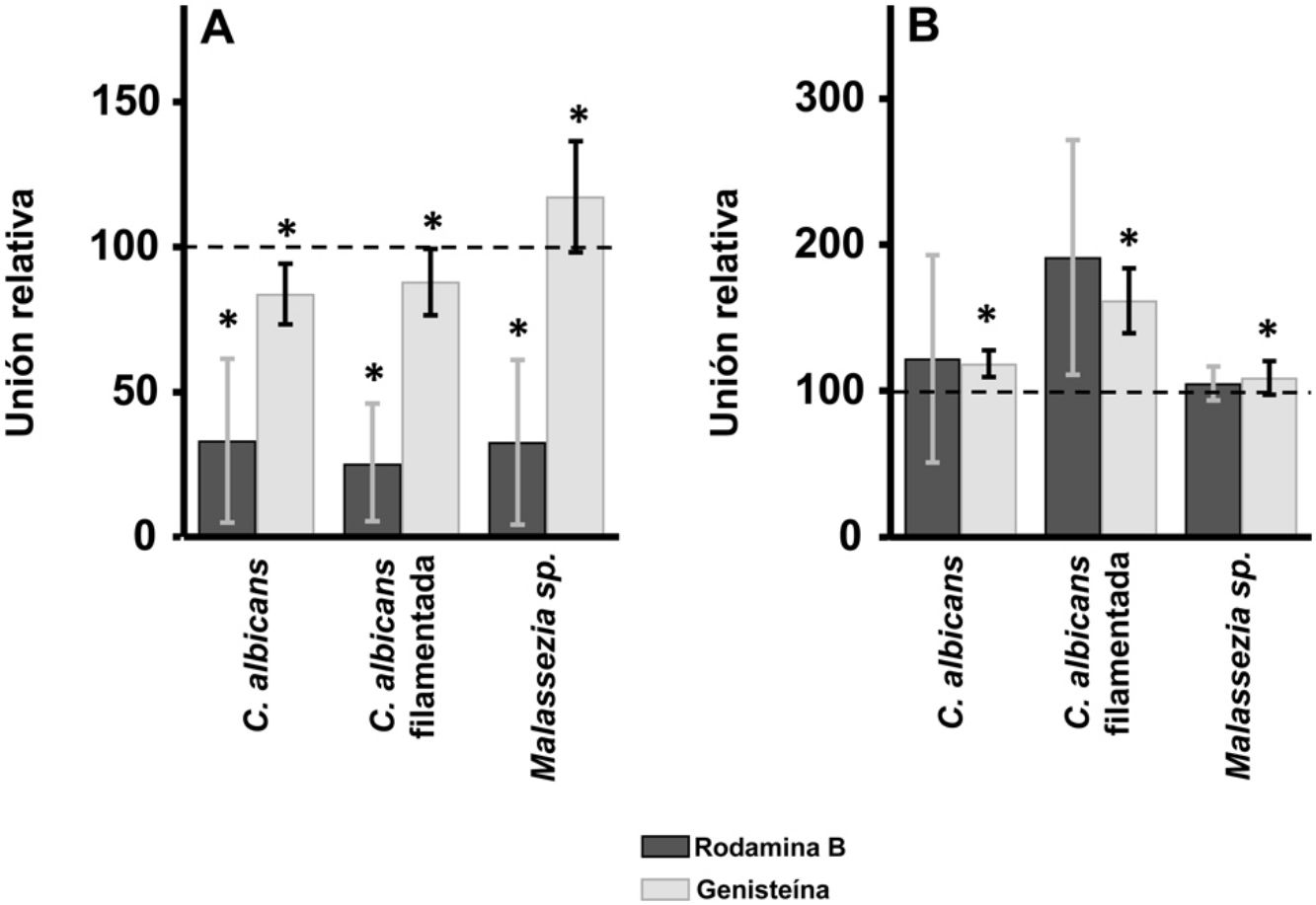
ResultadosEl tratamiento con rodamina B produjo una inhibición parcial de la adherencia de ambas especies fúngicas a queratinocitos, pero no a fibroblastos. La digestión selectiva del heparán sulfato produjo un aumento de la unión de Malassezia a los queratinocitos y de ambas especies a los fibroblastos. La digestión del condroitín sulfato redujo la unión de C. albicans en los queratinocitos, pero favoreció la unión de la forma filamentada de esta levadura en los fibroblastos.
ConclusionesLos GAG de superficie celular de queratinocitos parecen estar implicados en la adherencia de Candida y Malasezzia a la superficie celular. En los fibroblastos, por el contrario, su eliminación favorece la adherencia, sugiriendo la implicación de otro tipo de receptores.
The skin is colonized by a variety of microorganisms, including bacteria and, to a lesser extent, fungi, viruses, and parasites.1 Numerous studies have described the importance of bacteria and their role in both maintaining skin homeostasis and contributing to certain types of skin infection. However, little is known about the mycobiome, the population of fungi that inhabit the body. Like bacteria, many fungi can have pathogenic effects if they proliferate excessively or the host's immune response is altered. Superficial mycoses are among the most prevalent diseases in the world, and the most commonly detected opportunistic fungi in these types of infections are yeasts of the genera Malassezia and Candida, followed by other fungi such as dermatophytes.2–4
Of the fungi found on adult skin, the most abundant belong to the genus Malassezia. These yeasts obtain carbon exclusively from lipids, which they are unable to synthesize, and mainly colonize sebaceous areas of skin.2,5 They account for more than 90% of commensal fungi present on the skin. The most abundant species are Malasseziaglobosa, Malasseziarestricta, and Malasseziasympodialis. Despite their commensal nature, these species can also be associated with common skin disorders such as pityriasis versicolor and seborrheic dermatitis.2,5,6
The genus Candida is a heterogeneous group of commensal fungi found on the skin, nails, and mucous membranes of 70% of individuals. However, in immunodeficient individuals, and in conditions conducive to immune compromise, they can become pathogens, causing superficial and even systemic infections with high mortality rates.7–9 Although about 20 Candida species are pathogens in humans, the vast majority of recorded candidiasis cases are caused by Candidaalbicans. One characteristic of members of this genus is that they are polymorphic, existing in yeast form or as hyphae or pseudohyphae.10–12
Through the skin and mucosae the human body is continuously exposed to a broad range of pathogens. To colonize these surfaces pathogens must first anchor themselves to receptors present in the host. This is a critical step that involves specific recognition between molecules on the surface of the microorganism and those that comprise the receptor.13 There are several cell-surface molecules that act as binding mediators, including carbohydrates, lipids, proteins, and proteoglycans (PGs).14,15 PGs are made up of a central protein molecule to which varying numbers of polysaccharide chains called glycosaminoglycans (GAGs) are attached. GAGs consist of varying numbers of disaccharide repeats. There are different types of GAGs, with distinct chemical structures. Two examples are heparan sulfate (HS) and chondroitin sulfate (CS), which are formed by binding of glucuronic acid (GlcA) to N-acetylglucosamine (in the case of HS) or to N-acetylgalactosamine (in the case of CS).16 Subsequently, these chains undergo a series of post-transcriptional modifications that confer great structural diversity, enabling their involvement in numerous physiological and pathological processes.16,17 The interaction of GAGs with a broad range of ligands, including cytokines, growth factors, and enzymes, implicates them in processes such as cell adhesion and migration, regulation of morphogenesis, inflammation, and even cancer and various infectious processes.18–20 The literature describes many situations in which GAGs facilitate pathogen binding to the surface of eukaryotic cells and in some case help achieve invasion, internalization, and dissemination of the pathogen.21
This study investigates the role of GAGs as receptors in the process of adhesion of 2 species of commensal skin fungi, Calbicans and Malassezia species, both of which act as opportunistic pathogens in certain circumstances. The aim of the study was to determine whether GAGs are involved in the adhesion of these microorganisms to epidermal keratinocytes and dermal fibroblasts, and to examine the role in these interactions of the main GAG species present on the cell surface. The findings could help to expand current knowledge of the molecular bases of adhesion, and propose novel anti-infective strategies that block a key initial step of the pathogenic process: pathogen adhesion to target tissues. The results may have broader relevance given that adhesion is also a prerequisite for many pathogens to deliver virulence factors into host cells.
Material and MethodsCell lines, fungal strains, and culture conditionsThis research was carried out in accordance with the principles of the Declaration of Helsinki. Keratinocytes and fibroblasts were acquired from human biopsies from the tissue bank of the Community Blood and Tissue Center of Asturias after first obtaining written informed consent in accordance with Spanish laws on organ and tissue donation for research. Cells were cultured as previously indicated.22
Calbicans and Malassezia species strains were obtained from clinical isolates provided by the Hospital Universitario Central de Asturias. Calbicans was grown at room temperature in Sabouraud (Difco) medium (BD, MD, USA) with chloramphenicol at a concentration of 50μg/L. To obtain filamentous Calbicans, medium containing 0.1% glucose, 1% glycine, and 0.1% yeast extract at pH 7.5 was incubated at 37°C and 5% CO2 for 48hours. Malassezia species were cultured at 37°C in Sabouraud medium enriched with 1% olive oil and 1% Tween 20.
Fluorescence labelingAfter overnight incubation fungi were washed 4 times with PBS. Subsequently, the cultures were resuspended in a PBS solution containing 0.1mg/mL fluorescein isothiocyanate (FITC; Sigma Aldrich, MO, USA) at an A600 of 0.5 and incubated in darkness for 1hour at 37°C. Next, the cultures were centrifuged, washed 4 times with PBS to remove excess FITC, and resuspended in PBS at an A600 of 0.5.
Adhesion assaysAssays to evaluate fungal adhesion to cell monolayers were carried out in 24-well plates in which cell cultures were grown to 70–90% confluence. The culture medium was removed and the cells were washed twice with PBS and blocked with 10% fetal calf serum in Dulbecco modified Eagle's minimal essential medium (DMEM) (Gibco, Life Technologies, CA, USA) for 2hours at 37°C and 5% CO2. Subsequently, the wells were washed with PBS, 200μL of the suspension containing labeled fungi was added, and the volume was made up to 500μL with DMEM medium. The mixture was then incubated for 90minutes at 37°C and 5% CO2, after which the wells were washed twice with PBS to remove unbound fungi. Finally, the cultures were disaggregated using 1% SDS and the fluorescence of the attached fungi was measured with a LS55 fluorimeter (Perkin Elmer, MA, USA), using excitation and emission wavelengths of 488nm and 560nm, respectively. The values from the different experiments were normalized to adhesion values obtained in the absence of any treatment, which were assigned an arbitrary value of 100.
Inhibition of GAG synthesisCell cultures in 24-well plates grown to approximately 70% confluence were incubated overnight in DMEM containing 50μg/mL rhodamine B (Sigma-Aldrich) and 30μM genistein (Sigma-Aldrich) at 37°C. The cultures were washed twice with PBS, cell integrity was evaluated under a light microscope, and adhesion assays were carried out as described in the previous paragraph.
Enzymatic digestion of GAG on the cell surfaceHS in cell cultures was digested by incubation in DMEM medium containing a mixture of heparinase I and III (500mU/mL each; Sigma Aldrich) for 3hours at 37°C and 5% CO2. CS digestion was performed in the same way, using 250mU/mL chondroitinase ABC (Sigma Aldrich). Reactions were stopped by washing twice in PBS, cell integrity was assessed under a light microscope, and adhesion assays were then carried out as described above.
Statistical analysisAll experiments were performed at least 3 times with at least 3 replicates per group. All data were analyzed using the Statistica program (Statsoft Inc.; Tulsa, OK, USA). Differences in mean values between 2 samples were compared using the Mann-Whitney U test, with significance set at P<0.05.
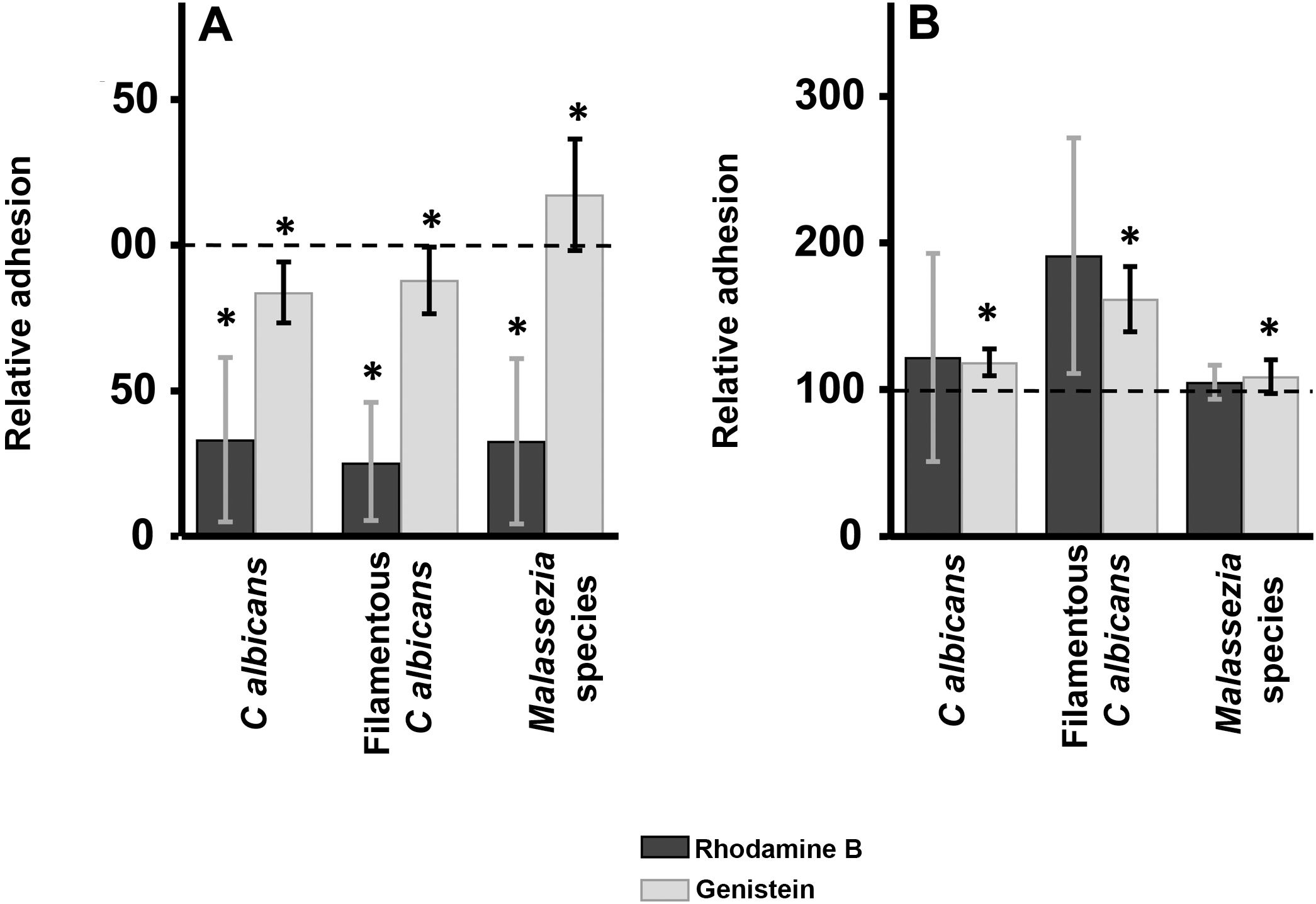
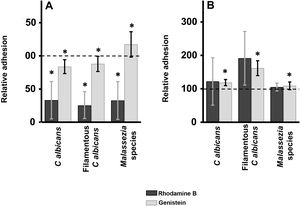
ResultsInhibition of GAG biosynthesis: effect on yeast epidermal keratinocytes and dermal fibroblastsTreatment of keratinocytes with either rhodamine B or genistein inhibited the adhesion of both yeast and filamentous forms of Calbicans. However, the intensity of the effect was dependent on the inhibitor. The strongest effect was observed for rhodamine B, while genistein only reduced adhesion values by a mean of 15% in both cases (Fig. 1A). In Malassezia species, rhodamine B had an effect similar to that observed for Calbicans, whereas treatment with genistein increased adhesion by around 17% compared with controls (Fig. 1A).
Effect of inhibition of GAG biosynthesis on yeast adhesion to skin cells. Graphs depict the effect of treatment with rhodamine B (dark bars) and genistein (light bars) on adhesion to keratinocytes (A) and fibroblasts (B). Data were normalized to yeast adhesion values recorded in untreated cells, to which an arbitrary value of 100 was assigned. Error bars represent standard deviation.
*P<0.05.
Addition of the same 2 inhibitors to dermal fibroblasts produced radically different results. Inhibition of adhesion was not observed in any case. Curiously, adhesion increased in all microbial forms analyzed. The increase in adhesion observed following genistein treatment was statistically significant (Fig. 1B).
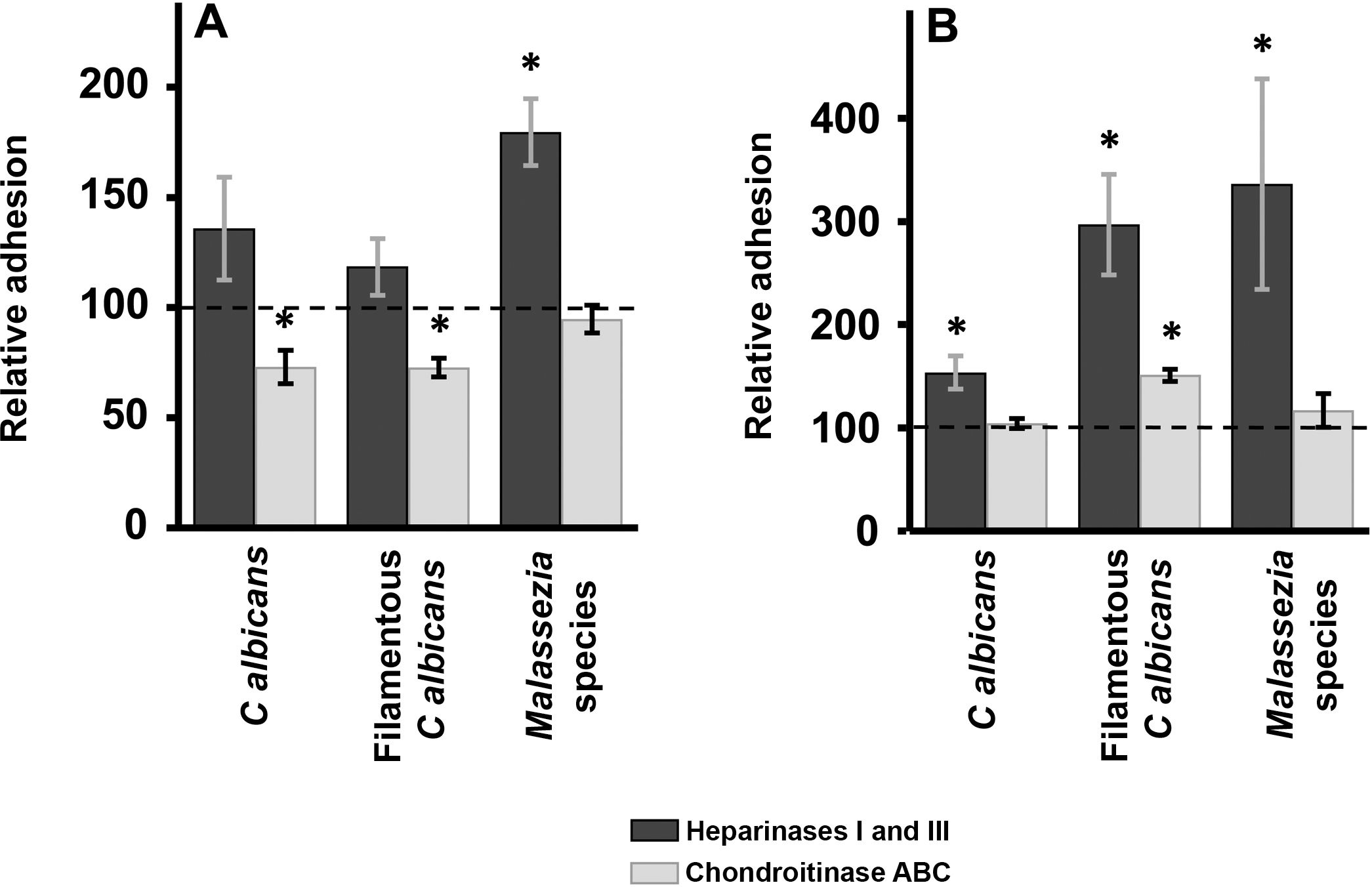
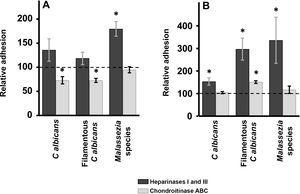
Enzymatic digestion of GAG: effect on yeast adhesion to epidermal keratinocytes and dermal fibroblastsTreatment of epidermal keratinocytes with heparinases I and III resulted in no significant differences in yeast adhesion with respect to controls, except in the case of Malassezia species, which showed an 80% increase in adhesion after elimination of HS from the surface of keratinocytes (Fig. 2A). Degradation of CS with chondroitinase ABC had the opposite effect: adhesion of Malassezia species was unchanged, but adhesion of both yeast and filamentous forms of Calbicans was significantly decreased (27% reduction in adhesion in both cases; Fig. 2A).
Effect of enzymatic degradation of cellular GAG on yeast adhesion to skin cells. Graphs depict the inhibitory effect of treatment with heparinases I and III (dark bars) or chondroitinase ABC (light bars) on yeast adhesion to keratinocytes (A) and fibroblasts (B). Data were normalized to yeast adhesion values recorded in untreated cells, to which an arbitrary value of 100 was assigned. Error bars represent standard deviation.
*P<0.05.
Digestion of HS cell on the cell surface of fibroblasts by treatment with heparinases I and III resulted in an increase in adhesion in all cases, an effect that was strongest for Malassezia species and was stronger for the filamentous versus the yeast form of Calbicans (Fig. 2A). Degradation of CS in fibroblasts resulted in no significant changes in adhesion with respect to controls, except in the case of the filamentous form of Calbicans, in which adhesion increased significantly (Fig. 2B).
DiscussionPGs in general and their GAG chains in particular are involved in different infectious processes, in which they act as receptors for many pathogens.21 The epidermis, the most superficial layer of the skin, is comprised mainly of keratinocytes, and its main function is that of a barrier that maintains homeostasis and protects against external aggressions including colonization and infection by pathogens. Immediately beneath the epidermis is the dermis, in which fibroblasts are found. This layer is less exposed to environmental factors and microorganisms. The participation of GAGs in microbial pathogenesis is conditioned by their structure,21 and this varies depending on the cell type and its physiological state.17 It is therefore of interest to investigate the role of these molecules as receptors in fungal infections.
To evaluate the involvement of GAGs in the interaction of Calbicans and Malassezia species with epidermal keratinocytes and dermal fibroblasts, we inhibited GAG synthesis with rhodamine B and genistein.
These inhibitors exerted distinct effects. In keratinocytes, rhodamine treatment markedly inhibited fungal adhesion. By contrast, genistein only mildly inhibited adhesion of cellular forms of Calbicans, and increased the adhesion of Malassezia species. Treatment of fibroblasts with the aforementioned inhibitors had no inhibitory effect on fungal adhesion, which was actually increased, in some cases significantly. However, the lack of significant differences was likely due to the variability of the data. The different effects of the inhibitors used are probably due to their distinct modes of action. Rhodamine B inhibits initiation and elongation of GAG chains,17,23,24 while genistein inhibits the activity of epidermal growth factor receptor kinase, which is required for complete expression of the genes that encode the enzymes involved in GAG production.25 However, it has been reported that the effects of genistein on GAG biosynthesis depend on GAG type and location.26,27 Taken together, these results show that GAGs appear to act as receptors for potentially pathogenic yeasts in keratinocytes, but not fibroblasts.
Enzymatic degradation of GAGs using different lysases showed that in keratinocytes CS appears to be more important for Calbicans adhesion, while elimination of HS promoted adhesion of Malassezia species. In dermal fibroblasts, no decrease in adhesion was observed after treatment with either inhibitor. In fact, in all cases increased adhesion was observed after elimination of HS from the cell surface. Elimination of CS by treatment with chondroitinase ABC promoted adhesion of filamentous Calbicans. Increased adhesion of yeast to skin cells after GAG degradation could favor greater exposure of another class of eukaryotic receptors involved in binding. On the other hand, the limited effect of degradation of individual GAG species on fungal adhesion could be indicative of cooperative phenomena involving both GAG species, as well as interaction with other receptors, as described in other epithelia.21
In conclusion, the present findings suggest that keratinocyte cell surface GAGs participate in Candida and Malassezia adhesion, probably in a cooperative manner. By contrast, in fibroblasts these molecules do not appear to play the same role and, in fact, interfere with adhesion, suggesting that adhesion is mediated by other receptors in these cells. The use of antifungal agents to treat these types of infections can lead to long-term resistance, underscoring the need for new therapeutic alternatives. The present findings open new avenues for the development of anti-adhesion strategies including competitive adhesin molecules that mimic GAGs, specific antibodies, and blockade of adhesin or cell receptor synthesis.
FundingThis work was funded by the 2019 ‘AEDV Investigates’ (‘AEDV investiga’) prize, awarded by the Healthy Skin Foundation(Fundación Piel Sana) of the Spanish Academy of Dermatology and Venereology (AEDV).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ordiales H, Vázquez-López F, Pevida M, Vázquez-Losada B, Vázquez F, Quirós LM, et al. Los glicosaminoglicanos se encuentran implicados en la adherencia de Candida albicans y Malassezia spp. a queratinocitos, pero no a fibroblastos dérmicos. Actas Dermosifiliogr. 2021;112:619–624.