Frontal fibrosing alopecia is an increasingly common form of scarring alopecia. The aim of this study was to describe the demographic and clinical characteristics of patients with FFA seen at the trichology unit of a medium-sized regional hospital and to report on treatments used.
Material and methodsWe reviewed the medical records of all patients with FFA seen at the trichology unit of Hospital Universitario Infanta Sofía in Madrid, Spain between May 2016 and May 2018. We analyzed associations between disease severity, clinical patterns, need for oral medications, and other characteristics.
ResultsSeventy-five patients (73 women and 2 men) were studied. Diagnosis was clinical in most cases and 13 cases (17.3%) were confirmed histologically. Median (interquartile range) age at reported onset of symptoms was 61 (12) years. Involvement of the eyebrows was recorded in 70 patients (93.3%) and signs of oral and genital lichen planus in 7 (9.6%). Eleven patients (14.7%) had hypothyroidism and 15 (20.0%) had signs of rosacea. Only 5 of the patients who presented a linear pattern (21.7%) had severe hairline recession. Patients with unstable and/or symptomatic disease (n=24) were treated with oral medications (5-alpha reductase inhibitors, hydroxychloroquine, corticosteroids, and isotretinoin) or intralesional corticosteroids. Eighteen patients (75.0%) achieved disease stability. Ten of the 15 patients with signs of rosacea and 10 of those with facial papules required systemic treatment.
ConclusionsMost of the patients in this series of FFA were postmenopausal women. The prevalence of oral and genital lichen planus was higher than that observed in the general population. Patients with a linear pattern had less severe disease. Facial papules were more common in younger patients and both facial papules and rosacea were associated with a greater need for oral treatment.
La alopecia frontal fibrosante (AFF) es un tipo de alopecia cicatricial cuya incidencia está en aumento. Detallamos las características demográficas, clínicas, y los tratamientos utilizados en los pacientes con AFF atendidos en la consulta de Tricología de un hospital de complejidad intermedia.
Material y métodoSe recopilaron de forma retrospectiva los datos de todos los pacientes diagnosticados de AFF atendidos en la consulta de Tricología del Hospital Universitario Infanta Sofía desde mayo de 2016 hasta mayo de 2018. Se evaluó la asociación entre la gravedad, el patrón clínico y la necesidad del tratamiento oral con el resto de las características de los pacientes.
ResultadosSe incluyeron un total de 75 pacientes (73 mujeres y 2 varones). El diagnóstico en la mayoría de los casos fue clínico, y se realizó estudio histológico en 13 pacientes (17,3%). La mediana de la edad de inicio del cuadro fue de 61 [12] años. En 70 casos (93,3%) se observó afectación de las cejas, y en 7 pacientes (9,6%) se objetivaron signos de liquen oro-genital. Asociaban hipotiroidismo 11 casos (14,7%), y en 15 (20,0%) se observaron signos de rosácea. Solo 5 (21,7%) de los pacientes con patrón lineal presentaban retroceso grave. En los pacientes inestables y/o sintomáticos (24 casos) se instauró tratamiento oral (con inhibidores de 5 alfa reductasa, hidroxicloroquina, corticoides o isotretinoína) o intralesional con corticoides, logrando la estabilización en 18 pacientes (75,0%). Un total de 10 pacientes de los 15 que presentaban signos de rosácea, y 10 de los 20 pacientes que presentaban pápulas faciales precisaron tratamiento sistémico.
ConclusiónLa mayoría de nuestros pacientes son mujeres postmenopáusicas. Hemos encontrado un aumento de la proporción de casos con un liquen orogenital en relación con la población general, y una menor gravedad en los pacientes con un patrón lineal. Se ha objetivado la presencia de pápulas faciales con más frecuencia en pacientes más jóvenes, y una mayor probabilidad de necesitar tratamiento oral en los pacientes con rosácea y con pápulas faciales.
Frontal fibrosing alopecia (FFA), first described by Kossard in 1994,1 is a type of scarring alopecia, the incidence of which appears to be increasing in recent years.
The demographic and clinical characteristics of FFA patients are described in several published cases series.2–17 This type of alopecia more frequently affects postmenopausal women, although it can also occur in premenopausal women and, less frequently, in men.18,19 It causes progressive and generally irreversible recession of the hairline, accompanied in many cases by loss of eyebrow hair.20 Signs of inflammation, such as erythema and peripheral flaking, are frequently observed around the hairline and the eyebrows. There are 3 described hairline recession patterns (diffuse, linear, and double line) that appear to have prognostic repercussions.16 Itching or burning sensations are reported by some patients. Facial papules and hair loss in other body areas, such as the axillae or the pubis, have been described in some cases.14
Diagnosis is primarily clinical, and can be supported by trichoscopic findings (peripheral erythema, follicular hyperkeratosis).21 In cases of atypical or ambiguous presentations it is necessary to take a skin biopsy for histopathological study. This type of alopecia is characterized by the presence of lymphocytic infiltrate and peripheral fibrosis.20 Some authors consider FFA a variant of lichen planopilaris, given the common pathological characteristics.
The etiology of FFA is unknown. Some authors have proposed an association between FFA and autoimmune diseases, including thyroid diseases22 and other dermatoses such as mucosal lichen planus4 and rosacea.23 Hormonal alterations have also been implicated in FFA, which predominantly affects postmenopausal women,20 although a recent study of women with FFA reported normal blood levels of sex hormones.24
The treatment objective is to stabilize the process to prevent progression of hairline recession and achieve symptom control. FFA treatments described in the literature include topical and oral corticosteroids, intralesional corticosteroids, topical immunomodulators, hydroxychloroquine, 5-alpha reductase inhibitors, and retinoids. However, few studies have compared the efficacy of multiple treatments.13,14
The aim of the present study was to describe the characteristics and prescribed treatments of FFA patients treated in a tertiary hospital providing intermediate care in the Community of Madrid, and to identify the factors associated with disease presentation, disease severity, and the need for oral treatment.
Material and MethodsWe designed an observational, descriptive, retrospective cross-sectional study of patients diagnosed with FFA and treated at the trichology unit of the Hospital Universitario Infanta Sofía (HUIS) between May 2016 and May 2018. Patients eligible for inclusion were those aged over 18 years with a clinical diagnosis of FFA (with or without histological confirmation) who had visited the trichology unit of the HUIS at least once during the study period. Diagnosis was based on the presence of scarring alopecia with recession of the frontotemporal hairline, bilateral diffuse alopecia of the eyebrows, and compatible trichoscopic signs. A biopsy was performed in cases of atypical clinical presentation.
Response variablesThe following demographic and clinical variables were collected for each patient: sex; age of alopecia onset; age at the moment of consultation; time since onset; severity (based on measurements taken at the first visit, considering severe FFA as frontal or lateral hairline recession ≥3cm); biopsy confirmation (yes, no); pattern (diffuse, lineal, double line, or other); smoking status (nonsmoker, smoker, ex-smoker); eyebrow involvement (yes, no), eyebrow involvement from onset (yes, no), loss of body hair (yes, no), mucosal lichen planus (yes, no), signs of rosacea (yes, no), facial papules (yes, no), hypothyroidism (yes, no), association with androgenetic alopecia (yes, no).
The treatment variables selected are shown below.
- -
Type of treatment (topical, systemic [including intralesional corticosteroids]). Patients with unstable FFA (i.e. hairline recession in preceding months and positive hair-pull test) and those presenting symptoms (pruritus, burning) were prescribed systemic treatment.
- -
Stabilization with systemic treatment (yes, no). Patients considered stable were those that showed no hairline recession, a negative hair-pull test, and symptom control during treatment follow-up visits (conducted every 3 months during and after treatment, during which comparisons were made with photographs taken prior to beginning treatment).
In the descriptive analysis, qualitative variables were expressed as absolute (n) and relative (%) frequencies, and quantitative variables as the mean±standard deviation or the median [interquartile range], depending on data normality as determined using the Shapiro-Wilk test.
Possible associations between the variables of interest were analyzed using the Chi-squared test or Fisher exact test for qualitative variables, and, in the case of quantitative variables, using the Student t test (or Mann Whitney U test) and ANOVA (or Kruskal Wallis test) for comparisons involving 2 and more than 2 study groups, respectively. Multivariate analyses (binary logistic regression) were performed to identify possible factors associated with FFA severity and the need for oral treatment.
A P value <0.05 was considered indicative of statistical significance. Data were analyzed using the Stata/IC program, v. 14 (StataCorp LLC., Texas, USA).
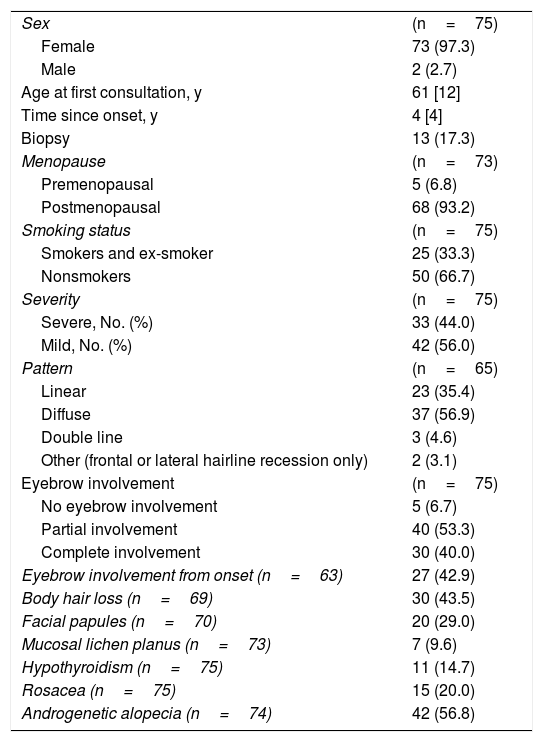
ResultsIn total, 75 patients (73 women and 2 men) fulfilled the inclusion criteria. The median age of self-reported symptom onset was 61 [12] years.
The results of the descriptive analysis together with patient clinical data are shown in Table 1.
Demographic and Clinical Characteristics of the Study Population
| Sex | (n=75) |
| Female | 73 (97.3) |
| Male | 2 (2.7) |
| Age at first consultation, y | 61 [12] |
| Time since onset, y | 4 [4] |
| Biopsy | 13 (17.3) |
| Menopause | (n=73) |
| Premenopausal | 5 (6.8) |
| Postmenopausal | 68 (93.2) |
| Smoking status | (n=75) |
| Smokers and ex-smoker | 25 (33.3) |
| Nonsmokers | 50 (66.7) |
| Severity | (n=75) |
| Severe, No. (%) | 33 (44.0) |
| Mild, No. (%) | 42 (56.0) |
| Pattern | (n=65) |
| Linear | 23 (35.4) |
| Diffuse | 37 (56.9) |
| Double line | 3 (4.6) |
| Other (frontal or lateral hairline recession only) | 2 (3.1) |
| Eyebrow involvement | (n=75) |
| No eyebrow involvement | 5 (6.7) |
| Partial involvement | 40 (53.3) |
| Complete involvement | 30 (40.0) |
| Eyebrow involvement from onset (n=63) | 27 (42.9) |
| Body hair loss (n=69) | 30 (43.5) |
| Facial papules (n=70) | 20 (29.0) |
| Mucosal lichen planus (n=73) | 7 (9.6) |
| Hypothyroidism (n=75) | 11 (14.7) |
| Rosacea (n=75) | 15 (20.0) |
| Androgenetic alopecia (n=74) | 42 (56.8) |
Data are presented as the median [interquartile range] or as n (%).
The key characteristics of the patient population are described below.
- -
A biopsy was performed for histological confirmation of diagnosis in 17.3% (n=13) of patients.
- -
Onset occurred after menopause in 93.2% (n=68) of female patients.
- -
Those who had never smoked accounted for 66.7% (n=50) of patients, while 33.3% (n=25) were active smokers or ex-smokers.
- -
Mild hairline recession was observed in 56.0% (n=42) of patients at the first consultation. The mean extent of hairline recession was 2.2±1.50cm.
- -
A diffuse hairline recession pattern was observed in 56.9% (n=37 cases) of patients.
- -
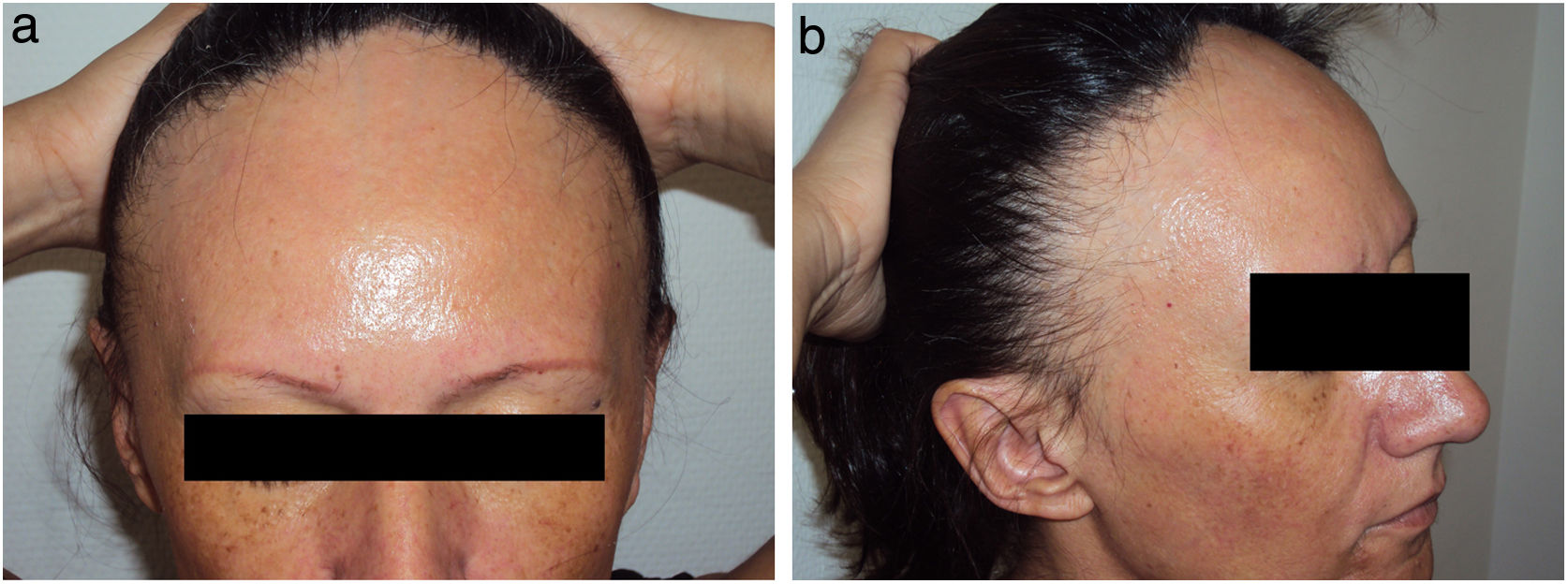
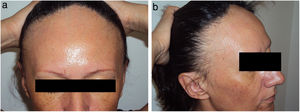
Data on the involvement of body areas other than the scalp revealed eyebrow involvement in 93.3% (n=70) of patients and facial papules in 29.0% (n=20) (Fig. 1).
- -
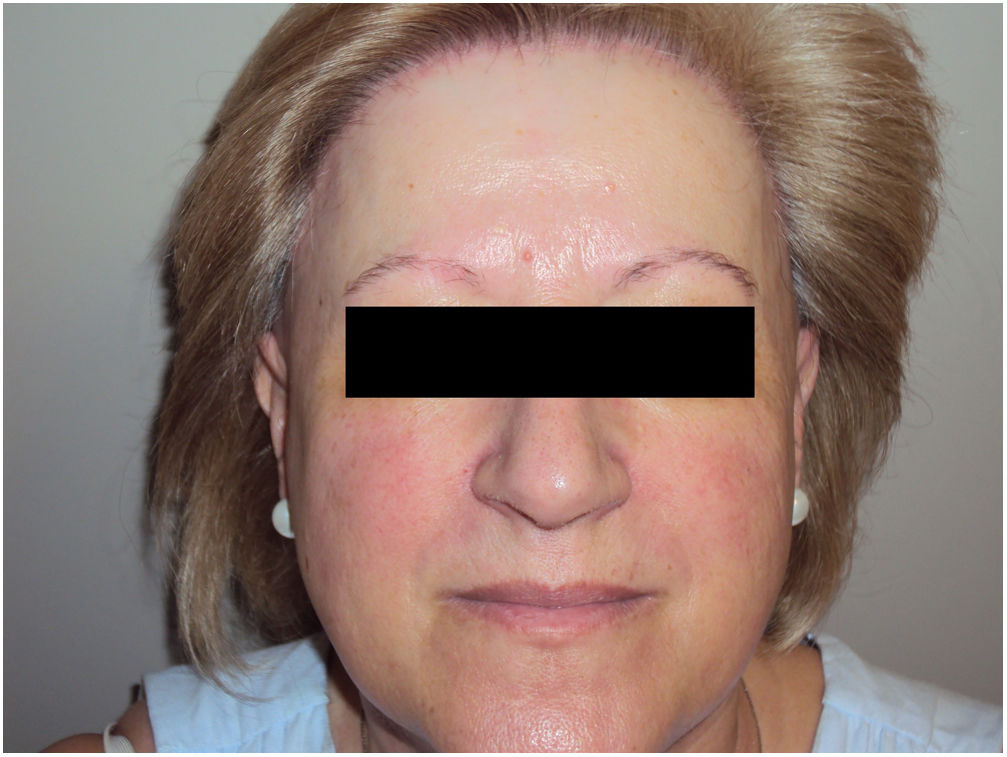
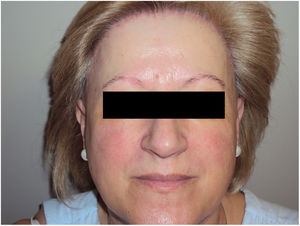
Mucosal lichen planus lesions were observed in 9.6% (n=7) of patients, hypothyroidism in 14.7% (n=11), and signs of rosacea in 20.0% (n=15) (Fig. 2).
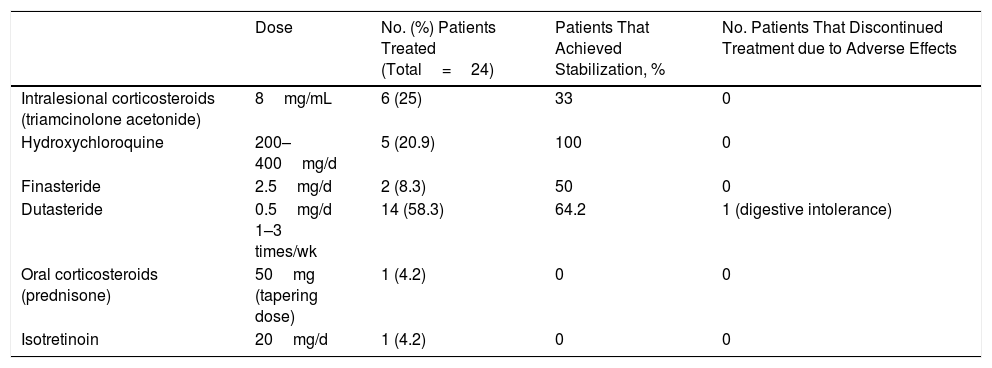
Systemic treatment (including intralesional corticosteroid therapy) was instituted in 32% (n=24) of patients (Table 2). The most commonly prescribed systemic treatment was 5-alpha reductase inhibitors, especially dutasteride (at 0.5mg 1, 2, or 3 times per week); 64.2% of patients treated with these compounds achieved stabilization lasting 8 to 18 months, except in 3 cases (1 was lost to follow-up, 1 opted to discontinue treatment, and 1 discontinued treatment due to a lack of efficacy after 4 months). Other treatments used were hydroxychloroquine, oral corticosteroids, isotretinoin, and intralesional corticosteroids (triamcinolone acetonide diluted in saline at a concentration of 8mg/mL).
Systemic Treatments Used, Percentage of Patients that Achieved Stabilization for Each Treatment, and Treatment Discontinuation due to Adverse Effects
| Dose | No. (%) Patients Treated (Total=24) | Patients That Achieved Stabilization, % | No. Patients That Discontinued Treatment due to Adverse Effects | |
|---|---|---|---|---|
| Intralesional corticosteroids (triamcinolone acetonide) | 8mg/mL | 6 (25) | 33 | 0 |
| Hydroxychloroquine | 200–400mg/d | 5 (20.9) | 100 | 0 |
| Finasteride | 2.5mg/d | 2 (8.3) | 50 | 0 |
| Dutasteride | 0.5mg/d 1–3 times/wk | 14 (58.3) | 64.2 | 1 (digestive intolerance) |
| Oral corticosteroids (prednisone) | 50mg (tapering dose) | 1 (4.2) | 0 | 0 |
| Isotretinoin | 20mg/d | 1 (4.2) | 0 | 0 |
The median [interquartile range] duration of treatment was 10 [7] months.
Stabilization of FFA was achieved in 75% (n=18) of the 24 patients who were prescribed systemic treatment, and was maintained in all cases during the follow-up period (median follow-up period after stabilization, 11.5 [9] months).
The results of the univariate analysis are presented in the tables in Appendix B (Supplementary Material, Tables 1–3).
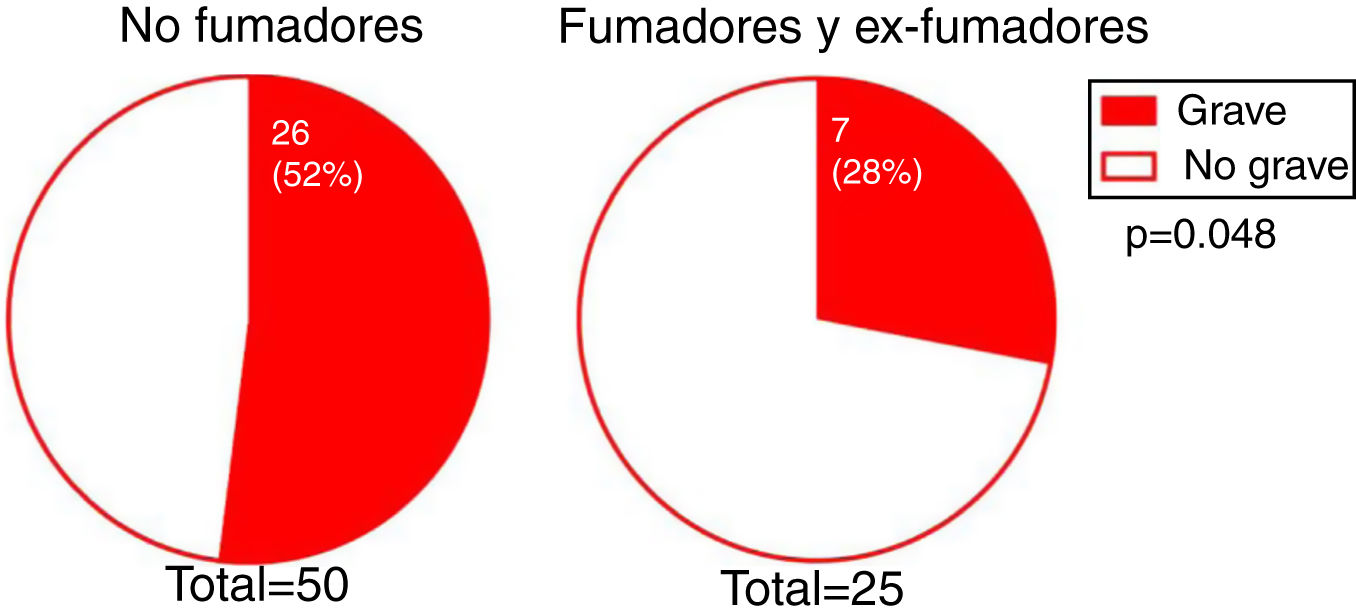
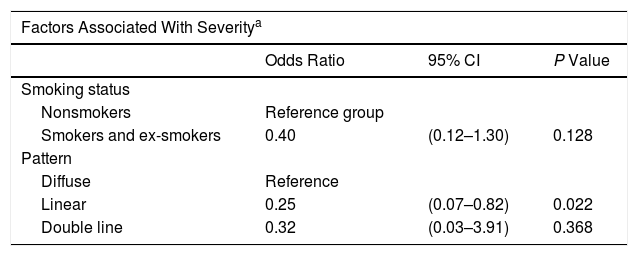
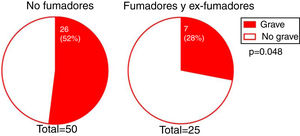
The degree of involvement was significantly greater in smokers or ex-smokers than in nonsmokers (P = 0.048) (Fig. 3). However, this association was not observed in the multivariate analysis (Table 3). No associations were observed between the severity of hairline recession and eyebrow involvement, eyebrow involvement from onset, body hair loss, the presence of facial papules, concomitant mucosal lichen planus, or signs of rosacea. Age at FFA onset did not differ significantly between patients with severe versus mild hairline recession. However, the median [interquartile range] time since onset tended to be longer in patients with severe versus mild hairline recession (4 [5] and 3 [3] y, respectively; P = 0.095).
Results of the Multivariate Analysis
| Factors Associated With Severitya | |||
|---|---|---|---|
| Odds Ratio | 95% CI | P Value | |
| Smoking status | |||
| Nonsmokers | Reference group | ||
| Smokers and ex-smokers | 0.40 | (0.12–1.30) | 0.128 |
| Pattern | |||
| Diffuse | Reference | ||
| Linear | 0.25 | (0.07–0.82) | 0.022 |
| Double line | 0.32 | (0.03–3.91) | 0.368 |
| Factors associated with the need for oral treatmentb | |||
|---|---|---|---|
| Odds Ratio (95% CI) | P Value | ||
| Facial papules | |||
| No | Reference group | ||
| Yes | 11.33 | (2.21–58.15) | 0.004 |
| Rosacea | |||
| No | Reference group | ||
| Yes | 4.25 | (1.08–16.71) | 0.038 |
| Rosacea×papules interaction | 0.24 | (0.02–3.12) | 0.272 |
Abbreviations: 95% CI, 95% confidence interval.
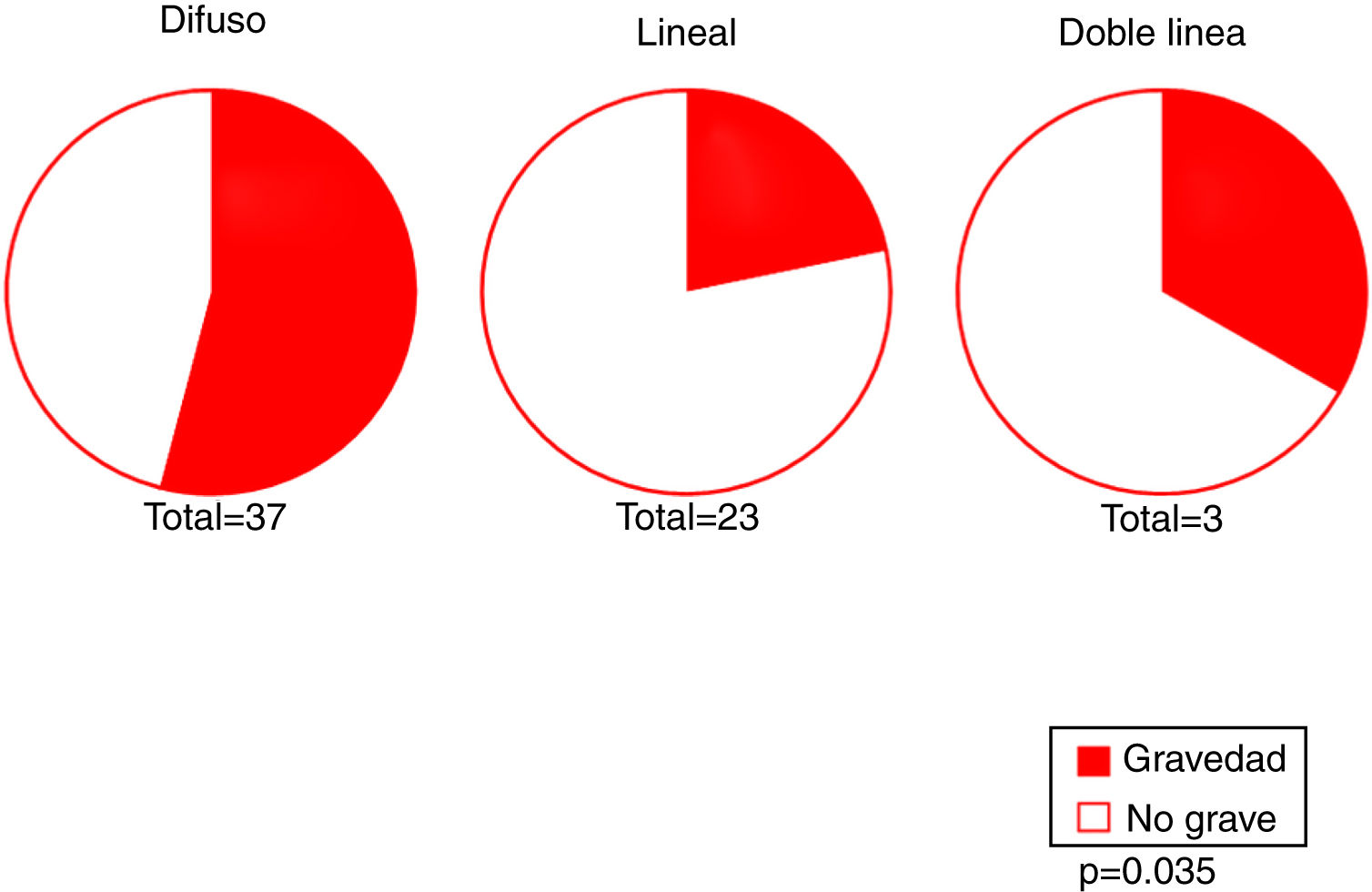
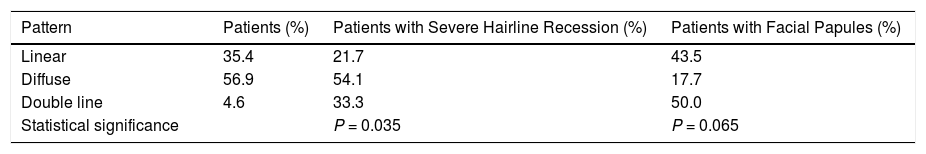
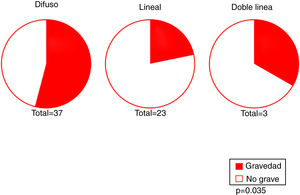
Our results revealed an association between the severity and pattern of hairline recession. Only 21.7% of patients with a linear pattern presented severe hairline recession. By contrast, for the other 2 patterns the proportion of patients with severe recession was significantly higher (P = 0.035). Logistic regression analysis showed that patients with a diffuse pattern were 4 times more likely to have severe hairline recession than those with a linear pattern (95% CI, 0.07–0.82; P = 0.022) (Fig. 4) (Table 3). Furthermore, the proportion of cases with facial papules differed between the different hairline recession patterns, accounting for only 17.7% of cases among patients with a diffuse pattern but greater proportions of patients with the other 2 patterns (P = 0.065) (Table 4). We observed no associations between the different hairline recession patterns and eyebrow involvement, body hair loss, concomitant mucosal lichen planus, or signs of rosacea. Furthermore, age at FFA onset did not differ significantly between patients with different patterns.
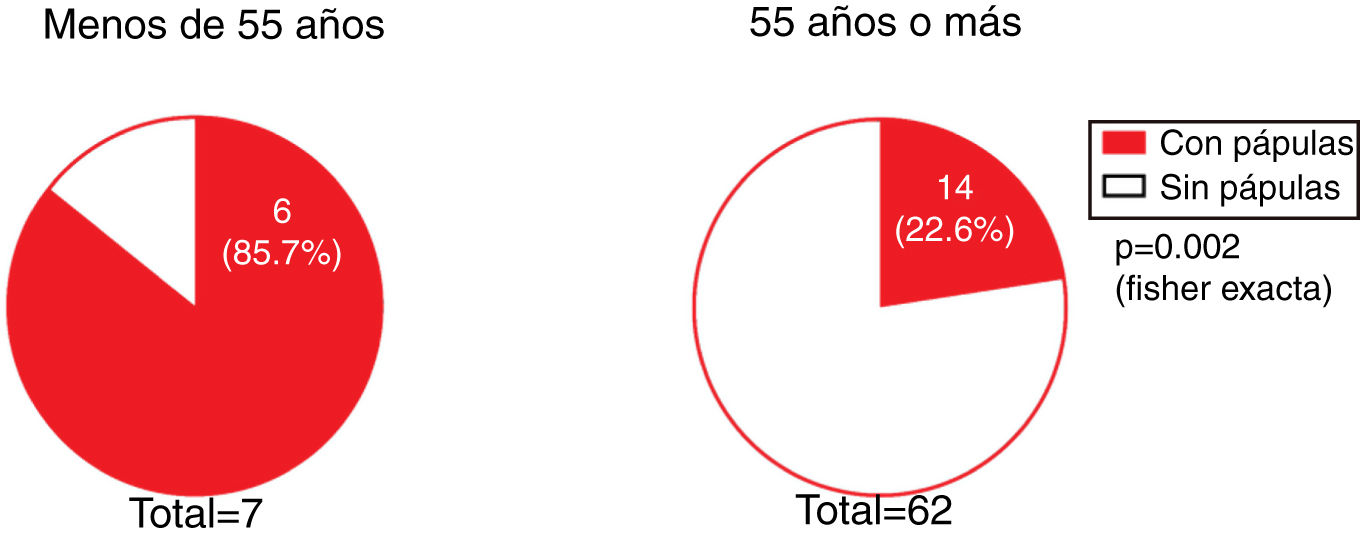
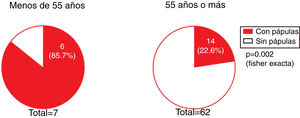
The presence of facial papules was significantly higher in patients under the age of 55 years than in those older (85.7% and 22.6%, respectively; P = 0.002) (Fig. 5).
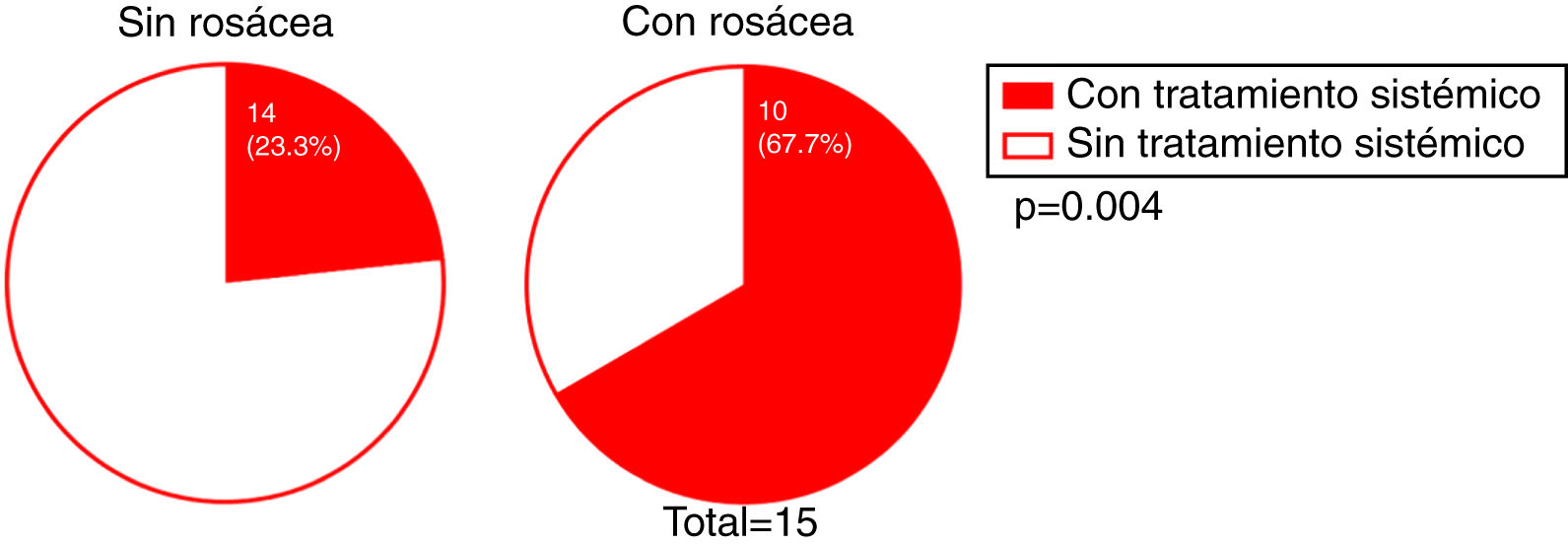
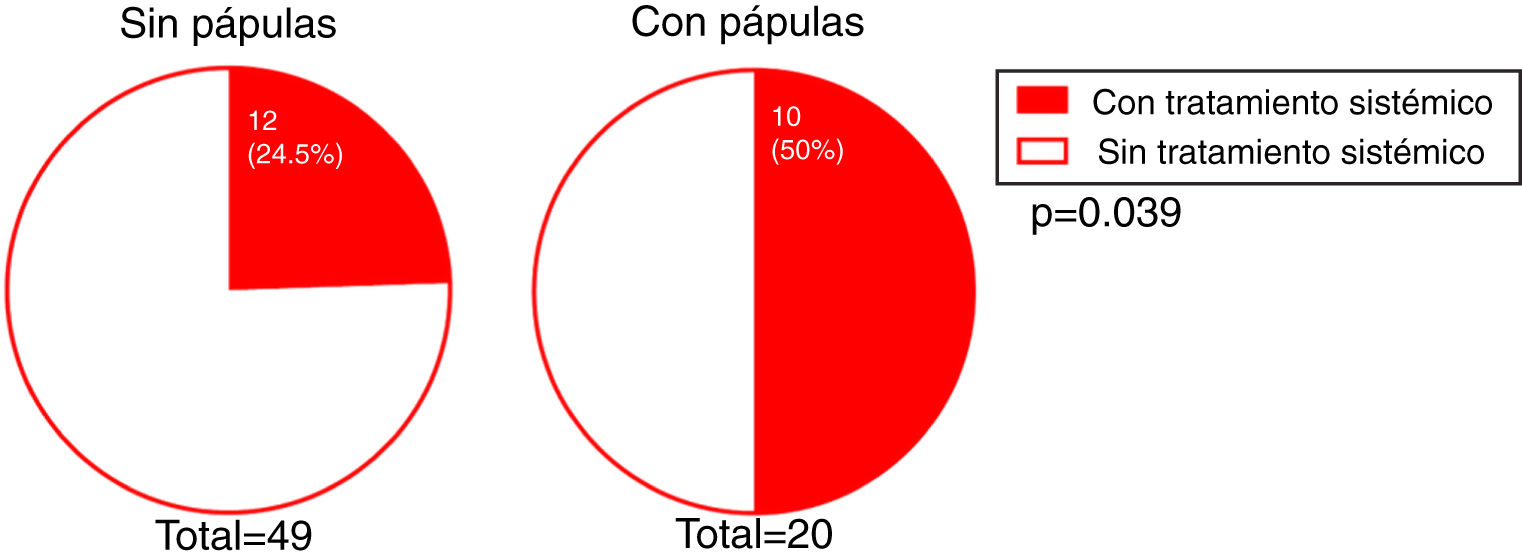
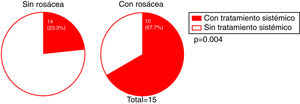
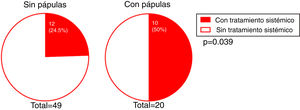
We investigated whether there was a correlation between the need for systemic treatment and any of the other variables included in our analysis. Our results show that the need for systemic treatment was significantly greater in patients with rosacea than those without (66.7% and 23.3%, respectively; P = 0.004) (Fig. 4) and in patients with facial papules than in those without (50% and 24.5%, respectively; P = 0.039) (Fig. 5). Logistic regression analysis confirmed that these variables were independently associated with the need for systemic treatment, which was 11.33 times greater (95% CI: 2.21–58.15; P = 0.004) in patients with rosacea than in those without, and 4.25 times greater in patients with papules than in those without (95% CI: 1.08–16.71; P = 0.038) (Table 3). We found no association between the need for systemic treatment and hairline recession pattern, eyebrow involvement, or concomitant mucosal lichen planus (Fig. 6).
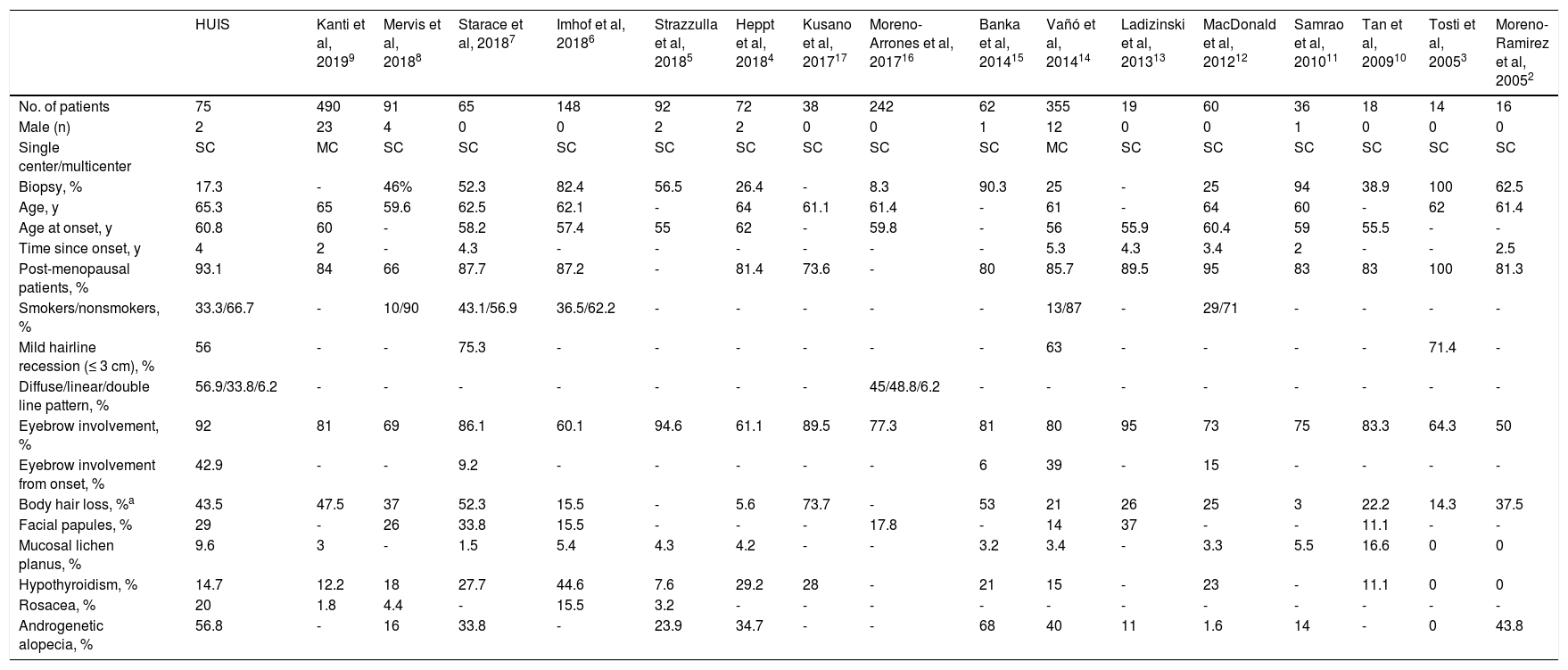
DiscussionFFA is a form of primary scarring alopecia that mainly affects women, especially those of postmenopausal age. The incidence of FFA appears to be increasing in recent years, and available treatments are relatively ineffective. Several published case studies have described the demographic and clinical characteristics of FFA patients (Table 5), and analyzed the outcomes achieved with different treatments (Fig. 7).
Frontal Fibrosing Alopecia: Comparison of Data from Present and Previously Published Series
| HUIS | Kanti et al, 20199 | Mervis et al, 20188 | Starace et al, 20187 | Imhof et al, 20186 | Strazzulla et al, 20185 | Heppt et al, 20184 | Kusano et al, 201717 | Moreno-Arrones et al, 201716 | Banka et al, 201415 | Vañó et al, 201414 | Ladizinski et al, 201313 | MacDonald et al, 201212 | Samrao et al, 201011 | Tan et al, 200910 | Tosti et al, 20053 | Moreno-Ramirez et al, 20052 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 75 | 490 | 91 | 65 | 148 | 92 | 72 | 38 | 242 | 62 | 355 | 19 | 60 | 36 | 18 | 14 | 16 |
| Male (n) | 2 | 23 | 4 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 12 | 0 | 0 | 1 | 0 | 0 | 0 |
| Single center/multicenter | SC | MC | SC | SC | SC | SC | SC | SC | SC | SC | MC | SC | SC | SC | SC | SC | SC |
| Biopsy, % | 17.3 | - | 46% | 52.3 | 82.4 | 56.5 | 26.4 | - | 8.3 | 90.3 | 25 | - | 25 | 94 | 38.9 | 100 | 62.5 |
| Age, y | 65.3 | 65 | 59.6 | 62.5 | 62.1 | - | 64 | 61.1 | 61.4 | - | 61 | - | 64 | 60 | - | 62 | 61.4 |
| Age at onset, y | 60.8 | 60 | - | 58.2 | 57.4 | 55 | 62 | - | 59.8 | - | 56 | 55.9 | 60.4 | 59 | 55.5 | - | - |
| Time since onset, y | 4 | 2 | - | 4.3 | - | - | - | - | - | - | 5.3 | 4.3 | 3.4 | 2 | - | - | 2.5 |
| Post-menopausal patients, % | 93.1 | 84 | 66 | 87.7 | 87.2 | - | 81.4 | 73.6 | - | 80 | 85.7 | 89.5 | 95 | 83 | 83 | 100 | 81.3 |
| Smokers/nonsmokers, % | 33.3/66.7 | - | 10/90 | 43.1/56.9 | 36.5/62.2 | - | - | - | - | - | 13/87 | - | 29/71 | - | - | - | - |
| Mild hairline recession (≤ 3 cm), % | 56 | - | - | 75.3 | - | - | - | - | - | - | 63 | - | - | - | - | 71.4 | - |
| Diffuse/linear/double line pattern, % | 56.9/33.8/6.2 | - | - | - | - | - | - | - | 45/48.8/6.2 | - | - | - | - | - | - | - | - |
| Eyebrow involvement, % | 92 | 81 | 69 | 86.1 | 60.1 | 94.6 | 61.1 | 89.5 | 77.3 | 81 | 80 | 95 | 73 | 75 | 83.3 | 64.3 | 50 |
| Eyebrow involvement from onset, % | 42.9 | - | - | 9.2 | - | - | - | - | - | 6 | 39 | - | 15 | - | - | - | - |
| Body hair loss, %a | 43.5 | 47.5 | 37 | 52.3 | 15.5 | - | 5.6 | 73.7 | - | 53 | 21 | 26 | 25 | 3 | 22.2 | 14.3 | 37.5 |
| Facial papules, % | 29 | - | 26 | 33.8 | 15.5 | - | - | - | 17.8 | - | 14 | 37 | - | - | 11.1 | - | - |
| Mucosal lichen planus, % | 9.6 | 3 | - | 1.5 | 5.4 | 4.3 | 4.2 | - | - | 3.2 | 3.4 | - | 3.3 | 5.5 | 16.6 | 0 | 0 |
| Hypothyroidism, % | 14.7 | 12.2 | 18 | 27.7 | 44.6 | 7.6 | 29.2 | 28 | - | 21 | 15 | - | 23 | - | 11.1 | 0 | 0 |
| Rosacea, % | 20 | 1.8 | 4.4 | - | 15.5 | 3.2 | - | - | - | - | - | - | - | - | - | - | - |
| Androgenetic alopecia, % | 56.8 | - | 16 | 33.8 | - | 23.9 | 34.7 | - | - | 68 | 40 | 11 | 1.6 | 14 | - | 0 | 43.8 |
In our series we specified the percentage of patients with axillary and/or pubic hair involvement. For all other series, values represent the percentage of patients with body hair involvement (if affected location was not specified), or the percentage of patients with axillary hair involvement alone or axillary and pubic hair involvement.
We present the results of a retrospective analysis of a series of 75 FFA patients who attended the trichology unit of the HUIS over a 2-year period (2016–2018).
In line with the findings of previous studies, the majority of patients (97.3%) were women, most of whom (93.2%) reported FFA onset after menopause.
Biopsy was required for diagnostic confirmation in only 17.3% of cases, in patients with incipient involvement of recent onset or atypical presentations (e.g. the absence of obvious eyebrow involvement).
More than half of our patients (56%) showed mild hairline recession at the time of consultation. Mild hairline recession has also been reported in the majority of subjects in other published series.3,7,14
We defined the different clinical patterns based on the series published by Moreno-Arrones et al.16 Those authors found that the linear pattern was the most frequent (48.8%). By contrast, in our series the diffuse pattern was observed in over half (56.9%) of all patients.
Our data on eyebrow involvement, body hair loss, and the presence of facial papules were similar to those reported in other series.7
In our study population, concomitant lichen planus was observed in 9.6% of patients. This rate is considerably higher than the prevalence of lichen planus in the general population (1–4%), suggesting a possible association between the 2 processes.
Other authors have proposed that FFA may be associated with hypothyroidism6,14,15 and with rosacea.6,23 In our series the prevalence of hypothyroidism (14.7%) and rosacea (20%) was strikingly high, far exceeding the estimated prevalence in the general population (4.2% and 2–5%, respectively). More than half (56.8%) of our patients also had androgenetic alopecia, in line with the findings of other Spanish series.2,14
In agreement with the results of previous studies,5,12 patients who received systemic treatments showed generally good outcomes: 75% of this group achieved stabilization of FFA. The most widely used systemic treatment was 5-alpha reductase inhibitors, in particular dutasteride. More than half (64.2%) of all patients treated with these agents achieved stabilization, a proportion similar to that reported in other series.3,13,14 Stabilization was maintained in all patients during the follow-up period.
Most of our patients were nonsmokers. Univariate analysis revealed a significant association between smoking and mild hairline recession, although this association was not confirmed in the multivariate analysis. In their study, Fonda-Pascual et al25 reported a higher prevalence of severe FFA among nonsmokers, suggesting a possible protective effect of tobacco against FFA progression. In their series, MacDonald et al12 reported a greater proportion of nonsmokers among their FFA patients than in the general population, although this observation was not corroborated by other authors.7,14
We observed a significant association between the linear pattern and reduced severity, as also reported by Moreno-Arrones et al,16 and a trend towards an absence of facial papules in patients with the diffuse pattern.
Furthermore, we observed an association between the presence of facial papules and younger age, as previously reported.8,26 The etiology of these noninflammatory facial papules is not well defined, although perifollicular inflammation of facial hair, sebaceous gland hypertrophy, and alterations of elastic fibers are thought to be involved. Because they are more frequently observed in premenopausal women, facial papules may constitute an early sign of FFA, and could be cause to suspect this process in young women with early signs of hairline recession.
In our series there was a significant association between the need for systemic treatment and the presence of rosacea and facial papules. The logistic regression model revealed that the effects of these 2 factors on the need for treatment were independent of one another. This may indicate a need for earlier and more intensive treatment in FFA patients with concomitant rosacea and/or facial papules.
Our study has certain limitations. Firstly, it is a retrospective study, and therefore the clinical evaluation of the patients was not carried out with the specific purpose of collecting data for this study. Furthermore, owing to short follow-up time after completion of treatment, it was not possible to determine whether the stabilization of FFA achieved by some patients was maintained in the long term. It should also be noted that no trichoscopy data were collected and that smoking-related data did not include the number of packets smoked per year or the number of years since quitting smoking.
In conclusion, in our series of FFA patients women of post-menopausal age predominated and eyebrow involvement was observed in most cases. The prevalence of mucosal lichen planus among FFA patients was higher than that described in the general population. We found that the likelihood of presenting facial papules was higher in younger patients; patients with a linear hairline recession pattern had less severe FFA; and the need for oral treatment was greater in patients with rosacea and facial papules.
Ethical DisclosuresPatients' personal data were processed in accordance with Royal Decree-Law 5/2018, of July 27 2018.
This study was approved by the research commission of the HUIS.
All patients provided verbal informed consent for the collection of data from their medical records, and were informed that these data could be used for clinical studies.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Maldonado Cid P, Leis Dosil VM, Garrido Gutiérrez C, Salinas Moreno S, Thuissard Vasallo IJ, Andreu Vázquez C, et al. Alopecia frontal fibrosante: estudio retrospectivo de 75 pacientes. Actas Dermosifiliogr. 2020;111:487–495.