Tinea pedis and onychomycosis are among the commonest fungal diseases in the world. Recently, there has been an increase in the numbers of fungal agents implicated in these conditions.
ObjectiveTo analyze the epidemiology of fungal foot diseases and to identify associated etiological factors in outpatients attending the Department of Dermatology of Charles Nicolle Hospital in Tunis, Tunisia.
Patients and MethodsOne hundred and forty eight patients were assessed for the presence of fungal foot diseases during the period between January and April 2009. The mean age was 41.5 years (range: 2–87 years) and sex ratio was 0.8. A complete dermatological examination was performed on all subjects, and specimens of the feet were taken from patients presenting signs of tinea pedis or onychomycosis for microscopy and fungal culture.
ResultsFungal foot infection was suspected in 71 subjects, and the diagnosis was confirmed in 67 cases (45.3%) by positive microscopy or culture. Older age and family history of mycosis were predisposing factors for foot fungal infection. The condition was caused by dermatophytes in 57.1% of cases and Candida species in 35.7%. Trichophyton rubrum and Candida parapsilosis were the predominant dermatophyte and yeast species, respectively.
Tinea pedis y onicomicosis se encuentran entre las enfermedades micóticas más frecuentes del mundo. Recientemente, está creciendo el número de agentes micóticos implicados en estas enfermedades.
ObjetivoAnalizar la epidemiología de enfermedades micóticas del pie e identificar los factores etiológicos asociados en pacientes externos vistos en el Departamento de Dermatología del Hospital Charles Nicolle en Túnez, la República Tunecina.
Pacientes y métodosSe evaluaron ciento cuarenta y ocho pacientes para la presencia de enfermedades micóticas del pie durante el periodo de Enero a Abril 2009. La edad media fue de 41,5 años (rango: 2 a 87 años) y la relación sexo 0,8. A todos los pacientes se les realizó un examen dermatológico completo, cogiendo, así mismo, muestras de los pies en los pacientes con signos de tinea pedis o onicomicosis para el estudio microscópico y cultivo para hongos.
ResultadosSe sospechó de una infección de los pies por hongos en 71 pacientes, y la diagnosis fue confirmada in 67 casos (45,3%), que fueron positivos por microscopia o por cultivo. Las edades mayores y antecedentes familiares de micosis fueron factores que predisponen a infección micótica del pie. La condición fue causada por dermatofitos en 57,1% de los casos y por las especies de Candida en 35,7%, siendo Trichophyton rubrum y Candida parapsilosis el dermatofito y la especie fúngica predominantes, respectivamente.
Fungal infection of the feet is very common, as the feet provide a favorable environment for fungal growth. Several studies have assessed the prevalence of tinea pedis and onychomycosis in different countries, as well as in specific population groups.
Tinea pedis accounts for more than half of all cases of dermatophytosis and onychomycosis for almost 50% of nail disorders. This high frequency makes those infections, although considered mild, a public health concern. Better understanding of the factors that promote fungal infection of the feet is therefore needed to identify effective preventive measures.
Dermatophytes and yeasts are the most frequent causes of foot mycoses; however, the distribution of the different species varies among geographic regions.
The aim of the present work was to evaluate the frequency of foot mycosis in a Tunisian hospital population and to study the epidemiological and mycological features of identified cases.
Patients and MethodsWe conducted a cross-sectional study in outpatients attending the Department of Dermatology of Charles Nicolle Hospital in Tunis, Tunisia between January and April 2009. Twice a week, around 10 outpatients were selected at random and examined by the same dermatologist. All patients included in the study provided informed consent. All subjects were interviewed to collect demographic data (age, sex, and occupation), history of chronic diseases (diabetes mellitus, psoriasis, hyperhidrosis, peripheral vascular disease, immune dysfunction, immunosuppressive drug treatment, and foot trauma), and specific data (physical activities, wearing of used shoes, ritual religious washing, and family history of mycoses). In addition, subjects were examined for any abnormalities of the toenails and feet.
Samples of skin scrapings and toenail clippings from lesions clinically consistent with fungal infections (intertriginous or hyperkeratotic lesions of the soles or toenail onyxis) were collected and sent to the Department of Parasitology in Charles Nicolle Hospital for direct examination and culture. A microscopic examination of all specimens was carried out in 30% KOH solution. The specimens were cultured in 2 plates, 1 containing Sabouraud-chloramphenicol dextrose agar and the other containing Sabouraud-chloramphenicol cycloheximide. Cultures were evaluated for growth after 48h, then once weekly for a month. The specimen was considered to be positive when microscopic examination or culture was positive.
We used χ2 test or Fisher's exact test for comparison of percentages. An α error of 0.05 was applied in all analyses. A multivariate analysis was also carried to identify independent factors associated with fungal foot infection.
ResultsA total of 148 patients were included in the study: 67 males (45%) and 81 females (55%). The mean age was 41.5 years (range, 2–87 years). Diabetes was reported in 22% of patients, 2.7% had psoriasis, 13% had palmoplantar hyperhidrosis, 8% had peripheral vascular disease, 3% had an autoimmune disease, and 5% were taking immunosuppressant drugs. A family history of mycosis was reported by 12 patients (8%). Regular sports activities were reported by 10% of patients, 51% practiced ritual washing, and 49% wore used shoes.
A total of 71 subjects (48%) had clinical evidence of fungal infection in their feet. Fifty-seven subjects (38.5%) had tinea pedis, the most commonly observed subtype was interdigital (91.2%), followed by hyperkeratotic (43.8%). We found both interdigital and hyperkeratotic infection in 35.1% of patients with tinea pedis. Onychomycosis was noted in 56 individuals (37.8%); the big toenail was the most frequently involved (89.3%). Forty-four patients had concomitant foot and toenail infection.
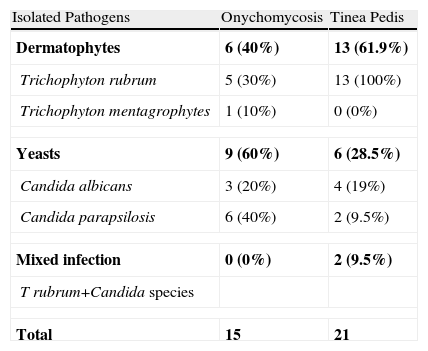
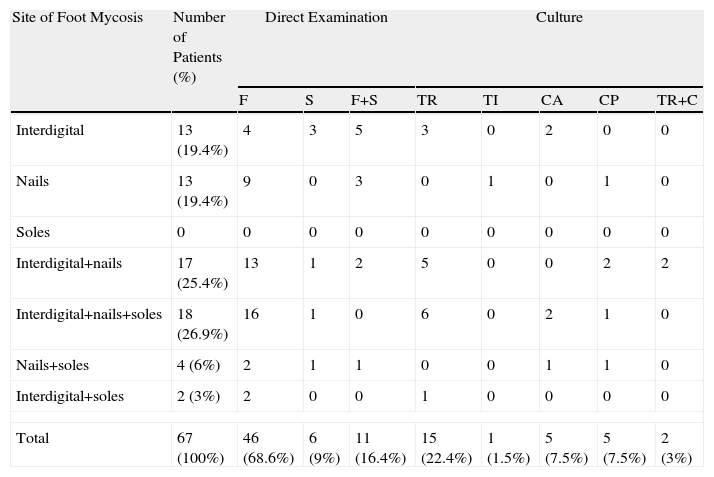
The fungal infection was confirmed by direct examination or culture in 67 subjects (45.3%). Direct microscopic examination was positive in 63 patients (88.7%) with clinical foot mycosis, showing filaments in 46 cases (73%), spores in 6 cases (9.5%), and both in 11 cases (17.5%). Culture was positive in 28 patients (41.8%), revealing the growth of dermatophytes in 16 cases (57.1%) and yeasts in 10 cases (35.7%). The predominant dermatophyte was Trichophyton rubrum (60.7%), followed by Trichophyton interdigitale (3.6%); the most commonly isolated yeast was Candida parapsilosis (25%) followed by Candida albicans (17.8%). Concomitant infection by Candida species and T. rubrum was seen in 2 cases. Percentages of the isolated fungi in the different sites are listed in Tables 1 and 2.
Percentages of Isolated Pathogens in Onychomycosis and Tinea Pedis.
| Isolated Pathogens | Onychomycosis | Tinea Pedis |
| Dermatophytes | 6 (40%) | 13 (61.9%) |
| Trichophyton rubrum | 5 (30%) | 13 (100%) |
| Trichophyton mentagrophytes | 1 (10%) | 0 (0%) |
| Yeasts | 9 (60%) | 6 (28.5%) |
| Candida albicans | 3 (20%) | 4 (19%) |
| Candida parapsilosis | 6 (40%) | 2 (9.5%) |
| Mixed infection | 0 (0%) | 2 (9.5%) |
| T rubrum+Candida species | ||
| Total | 15 | 21 |
Distribution of Mycosis According to Site.
| Site of Foot Mycosis | Number of Patients (%) | Direct Examination | Culture | ||||||
| F | S | F+S | TR | TI | CA | CP | TR+C | ||
| Interdigital | 13 (19.4%) | 4 | 3 | 5 | 3 | 0 | 2 | 0 | 0 |
| Nails | 13 (19.4%) | 9 | 0 | 3 | 0 | 1 | 0 | 1 | 0 |
| Soles | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Interdigital+nails | 17 (25.4%) | 13 | 1 | 2 | 5 | 0 | 0 | 2 | 2 |
| Interdigital+nails+soles | 18 (26.9%) | 16 | 1 | 0 | 6 | 0 | 2 | 1 | 0 |
| Nails+soles | 4 (6%) | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Interdigital+soles | 2 (3%) | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total | 67 (100%) | 46 (68.6%) | 6 (9%) | 11 (16.4%) | 15 (22.4%) | 1 (1.5%) | 5 (7.5%) | 5 (7.5%) | 2 (3%) |
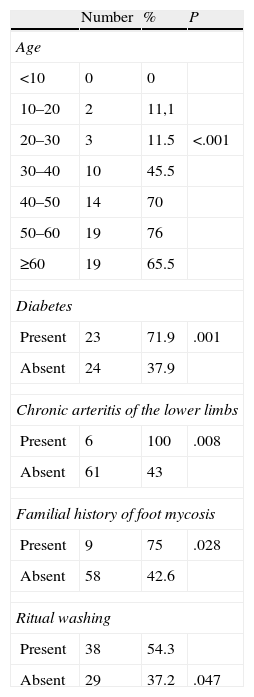
Among patients with confirmed foot mycosis, there were 34 males (50.75%) and 33 females (49.25%). The mean age was 52 years (range, 12–82 years), and the prevalence was highest in patients between 50 and 59 years (76%; P<.001). The highest prevalence of foot mycosis was noted in manual workers (70.8%) and retired persons (71.4%). There was a significant association between fungal foot infection and diabetes (P=.001), chronic arthritis of the lower limbs (P=.008), ritual washing (P=.047), and family history of foot mycosis (P=.028) (Table 3). However, in the multivariate analysis, only advanced age (over 50 years) and family history of mycosis were independently associated with fungal foot infection.
Epidemiological Data on the Group of Patients With Foot Mycosis.
| Number | % | P | |
| Age | |||
| <10 | 0 | 0 | |
| 10–20 | 2 | 11,1 | |
| 20–30 | 3 | 11.5 | <.001 |
| 30–40 | 10 | 45.5 | |
| 40–50 | 14 | 70 | |
| 50–60 | 19 | 76 | |
| ≥60 | 19 | 65.5 | |
| Diabetes | |||
| Present | 23 | 71.9 | .001 |
| Absent | 24 | 37.9 | |
| Chronic arteritis of the lower limbs | |||
| Present | 6 | 100 | .008 |
| Absent | 61 | 43 | |
| Familial history of foot mycosis | |||
| Present | 9 | 75 | .028 |
| Absent | 58 | 42.6 | |
| Ritual washing | |||
| Present | 38 | 54.3 | |
| Absent | 29 | 37.2 | .047 |
Our data have revealed that 45.3% of randomly selected individuals attending our dermatology department have evidence of fungal foot infection. Similar frequencies have been reported in Europe. In the Achilles project, a pan-European survey that included over 90000 patients, the prevalence of fungal foot infections was 40.6%.1 Nevertheless, in our study, the frequency of foot mycosis was higher than that reported in Algeria (19.9%),2 Libya (8.1%),3 Italy (23.4%),4 and Thailand (6%).5 This may be explained by the fact that our study included patients consulting in a tertiary care setting. Secondary spread of fungal organisms from the toe clefts to the nail has been proposed to explain the frequent association of tinea pedis with toenail onychomycosis.2 In our study, tinea pedis was associated with onychomycosis in more than half of all cases (61.9%). This rate was much higher than that found in Britain (20%),6 the USA (33.8%),7 and even in other Tunisian studies.8,9
The prevalence of onychomycosis and tinea pedis has been shown to increase with age. An predominance of fungal infections in older individuals has been reported in Europe,1 Tunisia,8 and the USA.10 On the other hand, fungal foot infections are found only rarely in children: only 0.18% of cases were observed in school children in Turkey11 and 0.4% in Nigeria.12 In our study, the frequency of tinea pedis was found to be higher in adults over 50 years of age. Some studies have reported a predominance of fungal foot infections in either males or females,10,13,14 but no differences were observed in our study. Fungal foot infections were also significantly more frequent when a family history of mycosis was present. Indeed, fungal transmission is facilitated by walking on carpets or bathroom floors, or by sharing shoes.25 Genetics has also been identified as a factor governing the epidemiology of onychomycosis; T. rubrum infection, for instance, shows a familial pattern of autosomal dominant inheritance.26 Other risk factors for foot mycosis that have been reported in the literature were initially found to be significant in our study. However, they did not remain as independently associated in the multivariate study. In fact, socio-professional status has been shown to be related to fungal foot infection, and manual workers and retired persons were most frequently infected in our study. In the literature, other populations are mentioned as at high risk of feet mycosis. These include coal-miners, soldiers,2 and athletes.1,15,16 Those results were explained by the prolonged use of occlusive footwear in those categories leading to maceration and repeated foot trauma. We did not notice a higher prevalence of foot mycosis in subjects who engaged regularly in sports activities, perhaps because professional athletes are seen in specialized medical institutions. Interestingly, we found a large proportion of subjects who practiced ritual washing among the cases of tinea pedis. The religious custom of washing the feet 5 times daily may be a cause of maceration and, thus, a risk factor for fungal infection. The spread of fungal organisms through contact with carpets in mosques and areas used for ritual washing has also been reported.17 Greater awareness is needed to avoid fungal contamination among people frequenting public swimming pools, Turkish baths, or mosques. Advice should be provided on measures such as avoiding walking bare foot and regularly cleaning carpets, as well as on the importance of treating foot mycosis.
Many chronic diseases have been linked to fungal foot infections. These include diabetes mellitus,18,19 psoriasis,20 hyperhidrosis,21 peripheral vascular disease,22,23 frequent foot trauma, HIV infection,24 and the use of immunosuppressive drugs. In our study, however, these conditions did not show significant association with the presence of fungal infection.
In our study, direct microscopic examination seems to be more sensitive than culture for microbiological diagnosis of dermatomycosis, as has been reported previously.27 The most frequently identified fungi were dermatophytes, followed by yeasts, and finally by the combinations of dermatophytes and yeasts. A predominance of dermatophytes has also been reported in Europe,1 the USA,28 and Canada.29 However, Lestringant et al.30 found a predominance of yeast in patients from Al-Ain (United Arab Emirates) with toe-web infection. The most commonly reported isolated fungi are Candida parapsilosis (in nails) and C. albicans (in tinea pedis) for yeasts, T. rubrum for dermatophytes, and Alternaria species for molds. T. rubrum is the most common dermatophyte in foot mycosis worldwide1–3,28,29,31; C. parapsilosis has been reported as the most common yeast causing onychomycosis in Algeria,2 Malta,32 and in another Tunisian study.33 Notably, non-dermatophyte filamentous fungi are now more frequently implicated in feet mycosis, especially in onychomycosis. They are mostly reported in tropical regions where they can be endemic (for instance, Scopulariopsis brevicaulis34–36), although Onychocola canadensis is isolated in colder areas.37
In summary, this study shows that fungal foot infections are common in Tunis. The frequency of tinea pedis complicated by onychomycosis was high. Older age and family history of mycosis are the main risk factors for foot mycosis in our population.
Conflicts of InterestThe authors declare that they have no conflicts of interest.