Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disorder with an enormous impact on the quality of life. Flares of AD are very common during pregnancy, presumably due to a shift from T helper (Th) 1 response to Th2 response during this period.1 Evidence on the efficacy and safety of systemic therapies for AD during pregnancy is very limited, and the European Task Force on Atopic Dermatitis (ETFAD) recommends that they should be restricted to corticosteroids, cyclosporine, and azathioprine.2 Dupilumab is an IgG4 antibody that specifically binds to the shared α chain subunit of the interleukin-4 (IL-4) and interleukin-13 (IL-13) receptors, thereby blocking the signaling of these two cytokines secreted by Th2 lymphocytes.3 This drug is expected to cross the placenta, similar to other IgG antibodies.1 We report a case of AD that was safely treated with dupilumab during pregnancy and breastfeeding.
We describe a 37-year-old woman who attended our hospital with an AD flare three years before pregnancy, with AD diagnosed since her first months of life. Her disease was mild until the age of 30 years. Previous flares were treated with topical corticosteroids (TCS), systemic corticosteroids (SCS), cyclosporine, and phototherapy. Her past medical history was significant for allergic rhinoconjunctivitis and an episode of eczema herpeticum two years before. At the time she began treatment with dupilumab, she had AD lesions affecting 22% of her body surface area (BSA), with an Eczema Area and Severity (EASI) score of 19.1, a SCORAD of 54.4, an Investigator Global Assessment (IGA) of 4 and an NRS pruritus of 7, resulting in a significant impact on her quality of life. The patient started dupilumab and experienced significant improvement, achieving EASI90 and a significant reduction of pruritus (NRS pruritus = 0) and IGA (IGA=1) after 12 weeks of treatment. The only reported adverse effect was mild arthralgias. After three years of treatment, the patient opted to conceive. Following consultations with both Dermatology and Obstetrician physicians, it was collectively decided to continue her treatment. There were no pregnancy complications, and AD remained controlled throughout the entire gestation period. She delivered a healthy full-term newborn. The patient continued the treatment during the 8-month breastfeeding period without experiencing any complications.
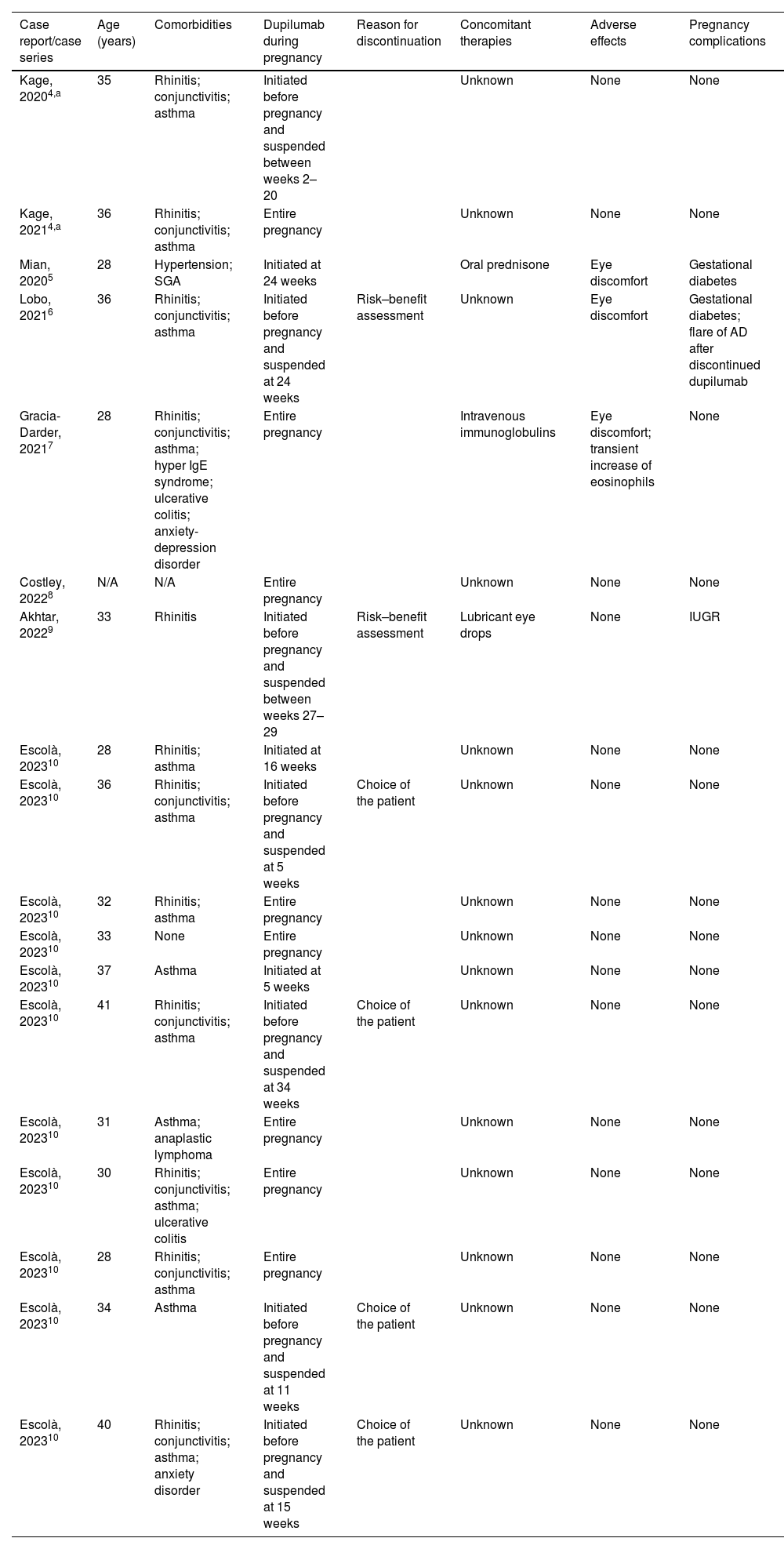
Managing of AD during pregnancy and breastfeeding can be challenging. Data on the safety of novel systemic medications is very limited. The European Medicines Agency (EMA) considered that the rate of spontaneous abortion registered during dupilumab studies did not seem to exceed the general rate.3 Data on the safety of the use of dupilumab for treating AD during pregnancy is limited to case reports and case series. We conducted a systematic review of all such reported cases in the literature to summarize existing evidence in Table 1.4–10 The MEDLINE database search was performed on 8 June 2023, with no filters applied. In a total of eighteen pregnancies (one patient had two pregnancies), there is one reported case of intrauterine growth restriction (IUGR). In this case, there were no anatomical or histological abnormalities in the placenta, and the infant was born with a mild low birth weight of 2480g. One pregnancy was complicated by a suspected small-for-gestational-age fetus before the patient started dupilumab, but the infant had an appropriate birth weight at delivery. Reported adverse effects include mild eye discomfort in 17% of patients, and one case of transient increase in eosinophils. Regarding lactation, there are eleven published cases of women who breastfeed their infants with different follow-up times, but no adverse events or complication were reported.4,10 Similar to other biologic agents with high molecular weight, the secretion of dupilumab in breast milk is minimal except during the first three days, when wide gaps between the breast alveolar cells allow immunoglobulins to pass through them.1
Summary of reported cases of use of dupilumab to treat Atopic Dermatitis in pregnancy.
| Case report/case series | Age (years) | Comorbidities | Dupilumab during pregnancy | Reason for discontinuation | Concomitant therapies | Adverse effects | Pregnancy complications |
|---|---|---|---|---|---|---|---|
| Kage, 20204,a | 35 | Rhinitis; conjunctivitis; asthma | Initiated before pregnancy and suspended between weeks 2–20 | Unknown | None | None | |
| Kage, 20214,a | 36 | Rhinitis; conjunctivitis; asthma | Entire pregnancy | Unknown | None | None | |
| Mian, 20205 | 28 | Hypertension; SGA | Initiated at 24 weeks | Oral prednisone | Eye discomfort | Gestational diabetes | |
| Lobo, 20216 | 36 | Rhinitis; conjunctivitis; asthma | Initiated before pregnancy and suspended at 24 weeks | Risk–benefit assessment | Unknown | Eye discomfort | Gestational diabetes; flare of AD after discontinued dupilumab |
| Gracia-Darder, 20217 | 28 | Rhinitis; conjunctivitis; asthma; hyper IgE syndrome; ulcerative colitis; anxiety-depression disorder | Entire pregnancy | Intravenous immunoglobulins | Eye discomfort; transient increase of eosinophils | None | |
| Costley, 20228 | N/A | N/A | Entire pregnancy | Unknown | None | None | |
| Akhtar, 20229 | 33 | Rhinitis | Initiated before pregnancy and suspended between weeks 27–29 | Risk–benefit assessment | Lubricant eye drops | None | IUGR |
| Escolà, 202310 | 28 | Rhinitis; asthma | Initiated at 16 weeks | Unknown | None | None | |
| Escolà, 202310 | 36 | Rhinitis; conjunctivitis; asthma | Initiated before pregnancy and suspended at 5 weeks | Choice of the patient | Unknown | None | None |
| Escolà, 202310 | 32 | Rhinitis; asthma | Entire pregnancy | Unknown | None | None | |
| Escolà, 202310 | 33 | None | Entire pregnancy | Unknown | None | None | |
| Escolà, 202310 | 37 | Asthma | Initiated at 5 weeks | Unknown | None | None | |
| Escolà, 202310 | 41 | Rhinitis; conjunctivitis; asthma | Initiated before pregnancy and suspended at 34 weeks | Choice of the patient | Unknown | None | None |
| Escolà, 202310 | 31 | Asthma; anaplastic lymphoma | Entire pregnancy | Unknown | None | None | |
| Escolà, 202310 | 30 | Rhinitis; conjunctivitis; asthma; ulcerative colitis | Entire pregnancy | Unknown | None | None | |
| Escolà, 202310 | 28 | Rhinitis; conjunctivitis; asthma | Entire pregnancy | Unknown | None | None | |
| Escolà, 202310 | 34 | Asthma | Initiated before pregnancy and suspended at 11 weeks | Choice of the patient | Unknown | None | None |
| Escolà, 202310 | 40 | Rhinitis; conjunctivitis; asthma; anxiety disorder | Initiated before pregnancy and suspended at 15 weeks | Choice of the patient | Unknown | None | None |
Abbreviations: AD, atopic dermatitis; IUGR, intrauterine growth restriction; SGA, small-for-gestation-age fetus.
In conclusion, our case adds to the existing reports of successful and safe use of dupilumab during pregnancy and lactation. However, due to lack of larger studies, it is not possible to recommend its use as a standard treatment approach. Currently, there are two observational studies registered in ClinicalTrials.gov (NCT03936335 and NCT04173442) that may help address this evidence gap. Considering that the current data suggests the safety of its use, it is reasonable to recommend it in severe cases of AD during pregnancy after a careful risk-benefit assessment and ensuring that these patients have appropriate surveillance by Dermatology and Obstetric physicians for early detection of potential adverse effects or complications.
Conflict of interestJosé Miguel Alvarenga and Ana Maria Lé have no conflict of interest. Tiago Torres has received consultancy and/or speaker's honoraria from and/or participated in clinical trials sponsored by AbbVie, Amgen, Almirall, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius-Kabi, Janssen, LEO Pharma, Eli Lilly, MSD, Mylan, Novartis, Pfizer, Samsung-Bioepis, Sanofi-Genzyme, Sandoz, and UCB.