Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the skin with a negative impact on quality of life. Up to now, there are no disease specific instruments in Spanish to assess quality of life in HS. The objective of this study was to develop and validate a questionnaire to evaluate the quality of life in patients with HS.
Material and methodsA multicentre study was carried out in Spain between 2016 and 2017 to develop the questionnaire. Both the conceptual framework and understanding of the patient's situation were considered through a review of the literature, consensus of professionals from different related health areas, and in-depth interviews with patients. The resulting questionnaire was passed to a group of 30 patients with 30±10 days of interval between both assessments.
ResultsThe reliability analysis shows a good internal consistency and reproducibility with Cronbach's alpha score of 0.920 (test) and 0.917 (retest) and intraclass correlation coefficient with DLQI and Skindex-29 of 0.698 95% CI (0.456-0.844) and 0.900 95% CI (0.801-0.951) respectively. Cut-off points were established for its use and the instrument was found to be sensitive to change.
ConclusionsThe HSQoL-24 is the first disease-specific self-administered instrument to assess quality of life in patients with HS in Spanish. It is user friendly, and easy to score. This study shows that the instrument is reliable, valid and sensitive to change, pending confirmatory study with a larger sample of 100 patients with HS.
La hidradenitis supurativa (HS) es una enfermedad inflamatoria crónica de la piel que influencia negativamente la calidad de vida. En la actualidad no existen escalas en español que la evalúen. El objetivo del presente estudio fue desarrollar y validar un cuestionario específico para evaluar la calidad de vida en pacientes con HS.
Material y métodosSe desarrolló un estudio multicéntrico en España entre 2016 y 2017 para elaborar un cuestionario. Para ello se consideró tanto el marco conceptual como el conocimiento de la situación del paciente mediante la revisión de la bibliografía, reuniones de profesionales de diferentes áreas y entrevistas con pacientes. El cuestionario resultante se pasó a un grupo de 30 pacientes con 30 ± 10 días de intervalo entre uno y otro.
ResultadosEl análisis de fiabilidad muestra una buena consistencia interna y reproductibilidad con puntuación alfa de Cronbach de 0,920 (test) y 0,917 (retest) y coeficiente de correlación intraclase con DLQI y Skindex-29 de 0,698 IC 95% (0,456-0,844) y 0,900 IC 95% (0,801-0,951) respectivamente. Se establecieron puntos de corte para su uso y se comprobó que el instrumento es sensible al cambio.
ConclusionesEl cuestionario HSQoL-24 es la primera prueba autoadministrada específica para evaluar la calidad de vida en HS en español. Sencillo de usar y puntuar por los profesionales. Este estudio demuestra que el instrumento es fiable, válido y sensible al cambio, pendiente de realizar estudio confirmatorio con una muestra mayor con 100 pacientes con HS.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease with negative physical and psychosocial effects.1,2
HS affects quality of life.3 Matusiak et al.4 analyzed areas of quality of life that were altered in patients with HS and found a marked effect on the patient-partner relationship, sexual relations, and family life. Suicidal ideation was recorded in some cases, and loss of work because of the disease was frequent.
The recent advent of new noninvasive procedures for early diagnosis of the disease5 and novel therapeutic targets will revolutionize our approach to HS.6 Therefore, given that HS is a very specific disease, this new scenario requires an instrument for measuring the quality of life of affected patients. However, our literature review showed that there was no such instrument in Spanish.
The objective of the present study was to develop a new psychometric questionnaire—the HSQoL-24—to evaluate the quality of life of patients with HS according to current development standards.
Material and MethodsThe study population comprised 30 adults with HS, all of whom signed the informed consent document. The study (PI16/020) was approved by the Clinical Research Ethics Committee of Aragon (CEICA) on February 10, 2016 and by the corresponding committees in the other hospitals. Patients were recruited at Hospital Royo Villanova (Zaragoza), Hospital Infanta Sofía (Madrid), Hospital Santa Creu i Sant Pau (Barcelona), and Hospital Doctor Negrín (Las Palmas de Gran Canaria).
Generation of ItemsFirst, we performed a literature review in PubMed in order to identify relevant psychosocial determinants in HS. These were used to identify areas for assessment in the interviews. Second, semistructured interviews were held with 12 patients (5 women and 7 men aged 18-61 years) who had been diagnosed with HS by a dermatologist. Third, once the interviews had been transcribed and coded, an expert panel was formed by 20 experts with broad clinical experience in order to generate new items. A 24-item psychometric instrument (HSQoL-24) was then drafted. The questions referred to the 4-week period preceding completion of the questionnaire, since the clinical course of HS is relatively stable over time compared with other skin conditions. The items were grouped into 6 domains: psychosocial, economic, occupational, relationships, personal, and clinical.
Psychometric EvaluationThe psychometric validation of HSQoL-24 was based on the Dermatology Life Quality Index (DLQI),7 which is a specific questionnaire for evaluating the quality of life of patients with skin conditions, and the Skindex-29 questionnaire, which had been adapted and validated in Spanish by Jones-Caballero et al.8 This is also used to evaluate the quality of life of patients with skin diseases.
Reliability was evaluated using internal consistency analysis with the Cronbach α (acceptable if> 0.7) and reproducibility analysis with the intraclass correlation coefficient (ICC) (adequate if> 0.7). Content validity was assessed based on the critical opinion of the 20 experts. Construct validity was assessed using a correlation and regression analysis with DLQI and Skindex-29. The cut points were calculated using receiver operating characteristic (ROC) curve analysis and comparison with the Skindex-29 categories. Sensitivity to change was analyzed based on correlations between differences in the scores for the 3 questionnaires and on effect size.
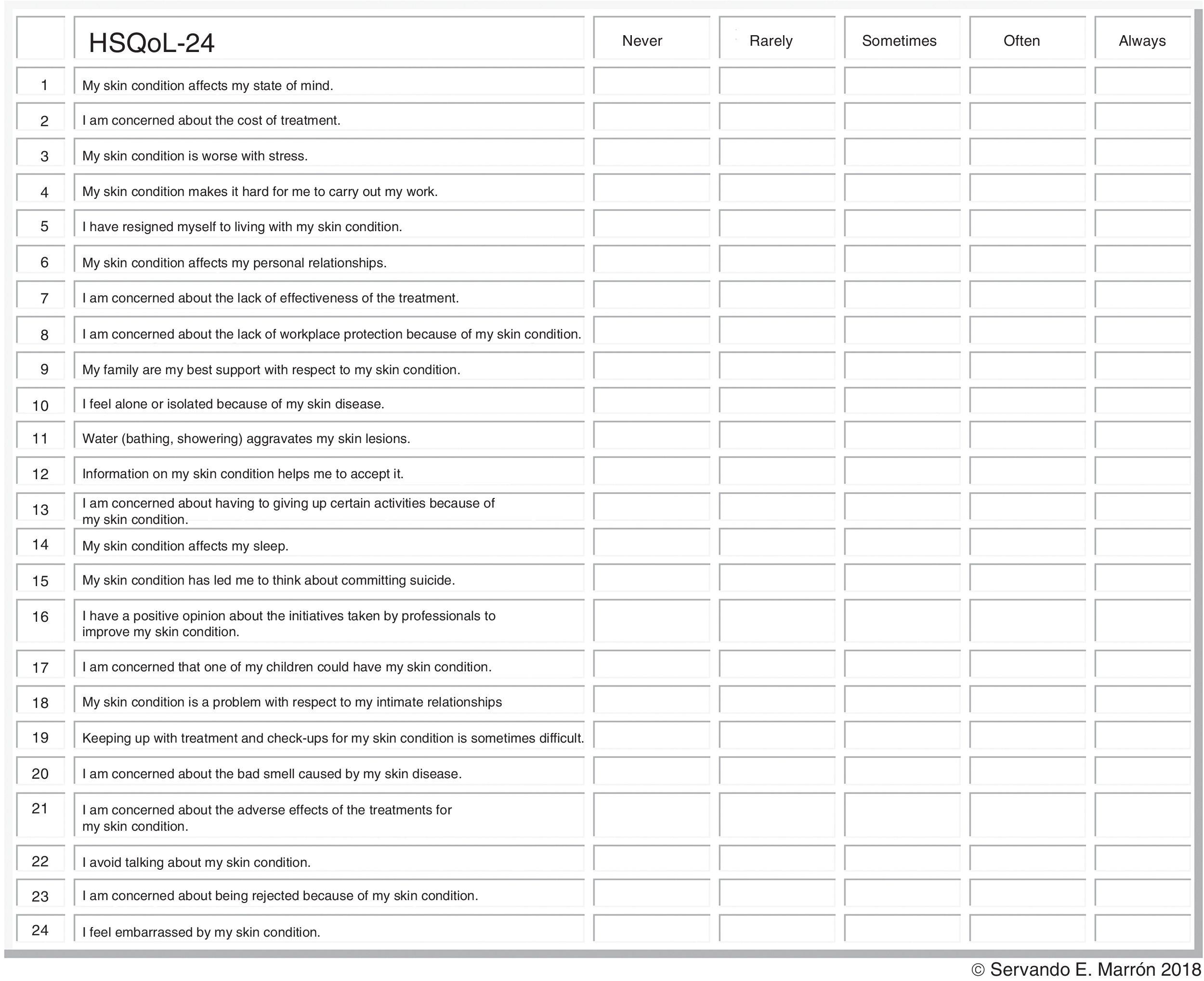
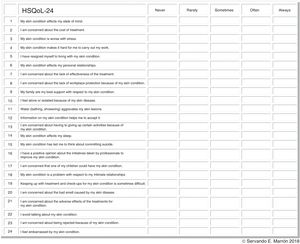
HSQoL-24 was developed by a group of experts from the Grupo Aragonés de Investigación en Psicodermatología (GAI+PD, Aragon Group for Research in Psychodermatology) and the Grupo Español de Investigación en Dermatología y Psiquiatría (GEDEPSI, Spanish Group for Research in Dermatology and Psychiatry) of the Academia Española de Dermatología y Venereología (AEDV, Spanish Academy of Dermatology and Venereology). An initial development phase was proposed in 30 patients—the object of the present study—with subsequent confirmation in 100 patients. The resulting questionnaire, HsQoL-24, with 24 items and 5 possible replies for each one (Fig. 1), proved to be feasible and easy to use by patients, who took approximately 10minutes to answer the questions.
ResultsThe HSQoL-24 was validated in the sample of 30 patients. The questionnaire was completed on 2 occasions with an interval of 30 ± 10 days between them. Eleven of the patients (36.7%) were men, mean (SD) age was 36.8 (11.2) years, age at onset was 22.8 (10.9) years, and time since onset was 13.6 (11.3) years. Six patients (20%) were in Hurley stage III, 14 (46.7%) in Hurley stage II, and 10 (33.3%) in Hurley I.
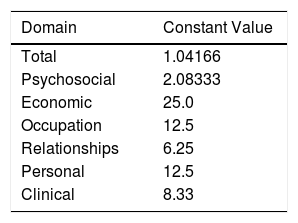
Properties of HSQoL-24The various items were grouped into 6 domains, as follows: psychosocial, items 1, 3, 5, 7, 10, 12, 15, 17, 20, 22, 23, and 24; economic, item 2; occupation, items 4 and 8; relationships, items 6, 9, 13, and 18; personal, items 11 and 14; and clinical, items 16, 19, and 21. When calculating the scores, it is necessary to remember that, as a rule, each item is scored from 0 to 4, as follows: 0, never; 1, rarely; 2, sometimes; 3, often; and 4, always. Items 9, 12, and 16 are scored inversely with respect to the others. Finally, the scores for each domain and the total score must be adjusted to 100, which is done by multiplying the value obtained by a constant (Table 1).
Value of Constants in Each Domaina
| Domain | Constant Value |
|---|---|
| Total | 1.04166 |
| Psychosocial | 2.08333 |
| Economic | 25.0 |
| Occupation | 12.5 |
| Relationships | 6.25 |
| Personal | 12.5 |
| Clinical | 8.33 |
The values for internal consistency (Cronbach α) were 0.920 in the initial evaluation (test) and 0.917 in the subsequent evaluation (retest).
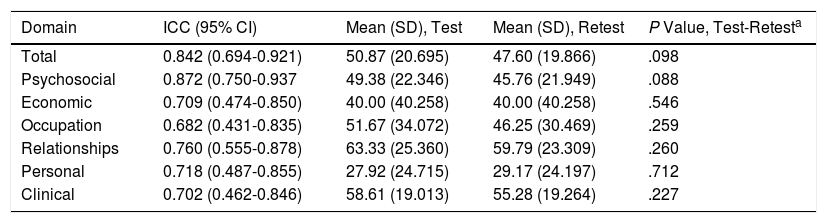
ReproducibilityReproducibility was measured using the ICC in a test-retest intervention. The evaluation was for total score and for the scores in the individual domains. The values of the ICC for DLQI and Skindex-29 were 0.698 (95% CI, 0.456-0.844) and 0.900 (95% CI, 0.801-0.951), respectively. The results are presented in Table 2.
Intraclass Correlation Coefficient.
| Domain | ICC (95% CI) | Mean (SD), Test | Mean (SD), Retest | P Value, Test-Retesta |
|---|---|---|---|---|
| Total | 0.842 (0.694-0.921) | 50.87 (20.695) | 47.60 (19.866) | .098 |
| Psychosocial | 0.872 (0.750-0.937 | 49.38 (22.346) | 45.76 (21.949) | .088 |
| Economic | 0.709 (0.474-0.850) | 40.00 (40.258) | 40.00 (40.258) | .546 |
| Occupation | 0.682 (0.431-0.835) | 51.67 (34.072) | 46.25 (30.469) | .259 |
| Relationships | 0.760 (0.555-0.878) | 63.33 (25.360) | 59.79 (23.309) | .260 |
| Personal | 0.718 (0.487-0.855) | 27.92 (24.715) | 29.17 (24.197) | .712 |
| Clinical | 0.702 (0.462-0.846) | 58.61 (19.013) | 55.28 (19.264) | .227 |
Abbreviations: ICC, intraclass correlation coefficient.
Construct validity was assessed using a correlation and regression analysis with DLQI and Skindex-29. Table 3 shows the values for the correlation between HSQoL-24 and DLQI and between HSQoL-24 and Skindex-29, both for the initial evaluation (test) and the subsequent evaluation (retest). The table shows how the correlation values are greater in the comparison with Skindex-29 and that they are close to 0.9 (0.863 and 0.898 for test and retest, respectively).
Correlation Analysis.
| Analysis, Test | Analysis, Retest | |||
|---|---|---|---|---|
| Correlation With DLQI (P Value) | Correlation With Skindex-29 (P Value) | Correlation With DLQI (P Value) | Correlation With Skindex-29 (P Value) | |
| Total | 0.708 (<.001) | 0.863 (<.001) | 0.691 (<.001) | 0.898 (<.001) |
Abbreviation: DLQI, Dermatology Life Quality Index.
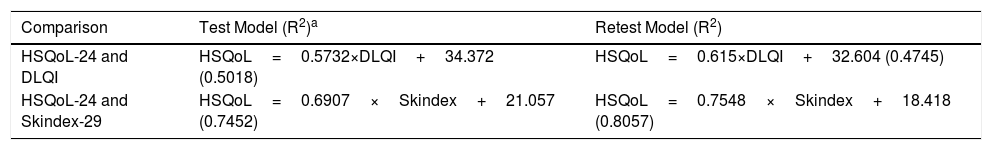
A regression analysis was performed for HSQoL-24 with respect to DLQI and Skindex-29 in the initial and subsequent evaluation. Table 4 shows the results of the initial evaluation (test). The R2 values for Skindex-29 revealed an adequate goodness of fit for the model (0.7452 and 0.7548 for test and retest, respectively). Therefore, DLQI makes it possible to predict 47%-50% of the value of variance in HSQoL-24; Skindex-29 shows a greater fit for the model, accounting for 75%-80% of the variance in HSQoL-24.
Regression Models.
| Comparison | Test Model (R2)a | Retest Model (R2) |
|---|---|---|
| HSQoL-24 and DLQI | HSQoL=0.5732×DLQI+34.372 (0.5018) | HSQoL=0.615×DLQI+32.604 (0.4745) |
| HSQoL-24 and Skindex-29 | HSQoL=0.6907×Skindex+21.057 (0.7452) | HSQoL=0.7548×Skindex+18.418 (0.8057) |
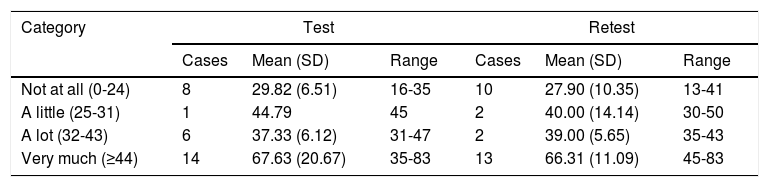
The cut points were delimited by comparing the range of values for HSQoL-24 and Skindex-29, since the latter yielded the best correlation and regression values. Table 5 shows the results of the descriptive study of the HSQoL-24 values in the different categories of Skindex-29. We can see that quality of life was not affected in only 8 and 10 patients and that it was severely affected in 14 and 13 patients.
Values of HSQoL-24 in Skindex-29 Categories.
| Category | Test | Retest | ||||
|---|---|---|---|---|---|---|
| Cases | Mean (SD) | Range | Cases | Mean (SD) | Range | |
| Not at all (0-24) | 8 | 29.82 (6.51) | 16-35 | 10 | 27.90 (10.35) | 13-41 |
| A little (25-31) | 1 | 44.79 | 45 | 2 | 40.00 (14.14) | 30-50 |
| A lot (32-43) | 6 | 37.33 (6.12) | 31-47 | 2 | 39.00 (5.65) | 35-43 |
| Very much (≥44) | 14 | 67.63 (20.67) | 35-83 | 13 | 66.31 (11.09) | 45-83 |
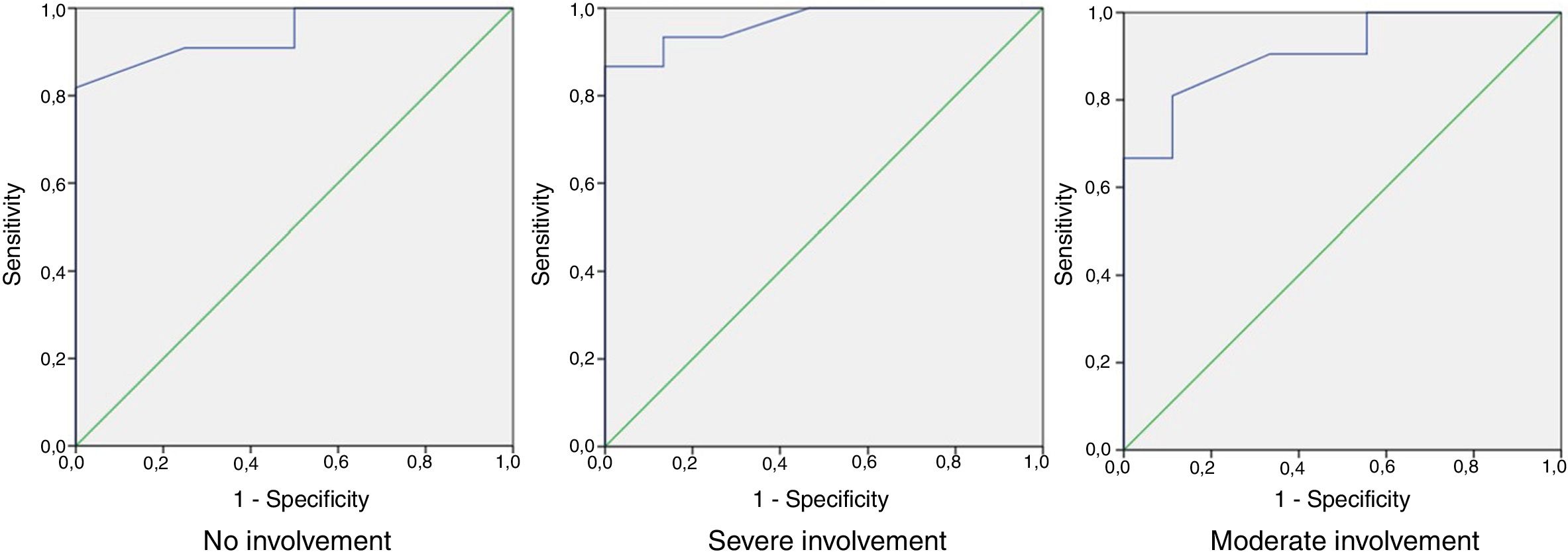
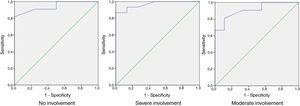
ROC curve analysis showed that values of between 0 and 32 in HSQoL-24 correspond to patients whose health-related quality of life (HRQoL) is not affected, with a sensitivity of 0.909 and a specificity of 0.5. The area under the curve was 0.943 (95% CI, 0.865-1.000). As for patients whose HRQoL was severely affected, the cut point of the proposed curve was 42, with a sensitivity of 0.933 and a specificity of 0.867. The area under the curve was 0.967 (95% CI, 0.909-1.000). The separation between the categories mild and moderate was proposed at a cut point of 36, with a sensitivity of 0.810 and specificity of 0.889 and an area under the curve of 0.910 (95% CI, 0.807-1.000) (Fig. 2).
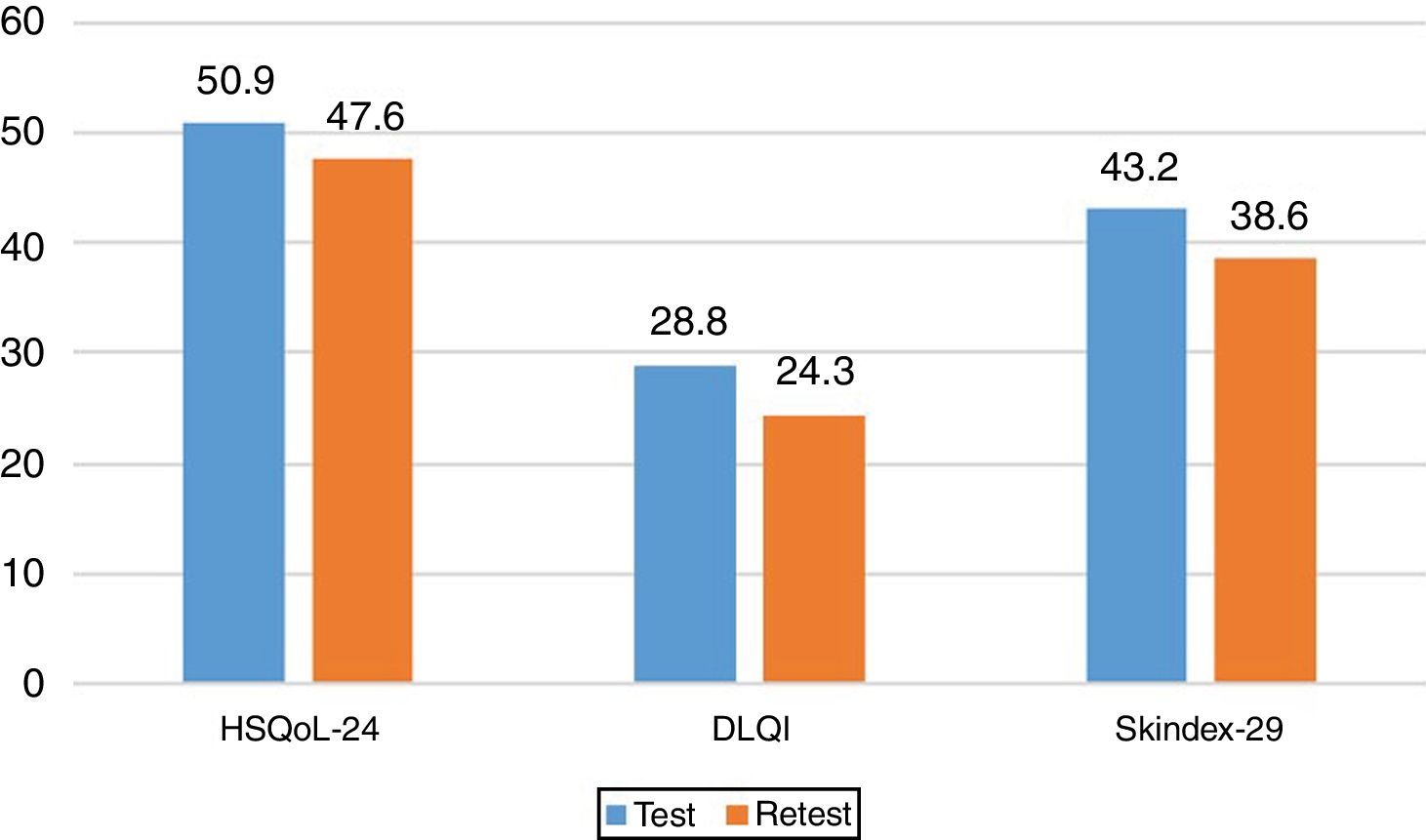
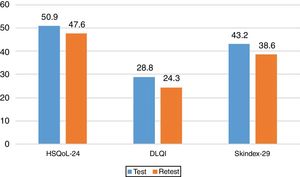
Sensitivity to ChangeSensitivity to change was assessed by calculating the variation in the 3 questionnaires and analyzing the correlations between them. The results revealed a statistically significant correlation between the change in HSQoL-24 and Skindex-29 (Pearson r=0.504, P=.004) and no statistically significant correlation for DLQI, although a tendency was observed (Pearson r=0.355, P=.055). The effect size for the variation in HSQoL-24, calculated as the difference in means divided by the deviation at baseline was 0.33 (50.87-47.60/20.695), thus indicating a reduced variation in HRQoL. Similar conclusions were reached for Skindex-29, with a value of 0.17 (43.2-38.6/25.865), thus indicating in both cases a slight variation in HRQoL in this type of patient in both questionnaires.
The values for the 3 questionnaires revealed differences in the total score and the final score. The mean values are shown in Fig. 3. We can see how these 3 mean values decreased.
DiscussionThe present article analyzes the characteristics of the HSQoL-24 questionnaire as a predictor of quality of life in patients with HS. During the drafting process, both the conceptual framework and awareness of the patient's situation were taken into account by means of a literature review, meetings between specialists from different areas, and interviews with patients. This working methodology is consistent with that of other authors and standard practice in the development of quality of life questionnaires.9,10
The relevance of evaluating quality of life in HS has been highlighted in several studies. Patients see their quality of life affected beyond their clinical manifestations, and this decline should be analyzed,11,12 as should associated factors.13 In addition to relevance, it is also important to generate questionnaires that are applicable to specific populations, in this case patients with HS in Spain, thus justifying the need for such research in the study of this disease today. Currently available questionnaires on HS are not applicable to the Spanish population until they are translated and validated. Moreover, instruments of this type had not been published at the time of the design of and subsequent data collection in the present study. Consequently, we did not envisage a translation of these questionnaires but instead opted to draft a completely new one.9,14,15
The psychometric characteristics analyzed in the present study were reliability, validity, and prognostic ability, which are equally well accepted in diseases that affect quality of life in Spain.16
Reliability was measured in terms of internal consistency and/or reproducibility/repeatability. Internal consistency was measured using the Cronbach α.17 The results revealed a value of> 0.9, thus indicating excellent internal consistency, which was maintained even when some of the questions were eliminated. Similar values were reported by McLellan et al.,16 namely, 0.955 in physical function and 0.900 in the symptom domain in a quality of life questionnaire in HS. Pinnard et al.15 also reported high values (0.936).
Reproducibility, or repeatability, was measured using the ICC in a test-retest intervention, assuming that values> 0.7 were adequate. This evaluation was performed for the total score and for the scores in the individual domains. The ICC values exceeded 0.7 in all domains except for occupation (0.68). These values are similar to those in other questionnaires or methods used in dermatology, for example, the computer-guided calculation of the Psoriasis Area and Severity Index (PASI), which reached values of 0.86 (95% CI, 0.80-0.90).18
The different ICC values in the DLQI and Skindex-29 overlapped considerably with the 95% CI of the ICC in HSQoL-24. Therefore, we can deduce that the ICC values of the 3 questionnaires were similar.
Construct validity was determined using correlation and regression analysis with DLQI and Skindex-29. High correlation coefficient values and appropriate fit of regression models revealed adequate validity, with a greater correlation (r> 0.85) and better goodness-of-fit (R2> 0.7). As reported elsewhere,15,16 HSQoL-24showed adequate correlations with these questionnaires, thus indicating adequate validity.
The cut points had adequate sensitivity (0.88 and 0.90) and a high area under the curve (> 0.9) in the ROC curve analysis, thus indicating adequate predictive capacity for detecting individuals with altered HRQoL. These values were similar to those reported by other authors for other HS and dermatology questionnaires.19,20 Nevertheless, it was not possible to calculate specificity values appropriately, probably because of the small sample size; therefore, in the next stage of the evaluation, we will be able to perform a more coherent analysis of the sensitivity and specificity values of HSQoL-24.
In addition to the parallel variations in test and retest quality of life scales commented on above, sensitivity to change was analyzed based on the study of correlations between differences in the questionnaire scores and on effect size. The results showed a statistically significant correlation between change in HSQoL-24 with respect to Skindex-29, although not DLQI, with a low P value (.055). Correlation values of around 0.5 were significant and slightly lower than in other dermatology questionnaires such as the Urticaria Activity Score, which, in its validation phase reached 0.6-0.7 with respect to other questionnaires.21 Nevertheless, the results for HSQoL-24 were considered indicators of good sensitivity to change.
ConclusionsThe results of the analysis of the psychometric properties of HSQoL-24, the first specific self-administered test in Spanish for evaluation of quality of life in patients with HS, revealed adequate reliability and validity values. Therefore, while awaiting the validation results for 100 patients, these findings make HSQoL-24 a very clinically valid instrument in terms of the results perceived by patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Marrón SE, Gómez-Barrera M, Tomás-Aragonés L, Díaz Díaz RM, Vilarrasa Rull E, Madrid Álvarez MB, et al. Desarrollo y validación preliminar del instrumento HSQoL-24 para evaluar calidad de vida en pacientes con hidradenitis supurativa. Actas Dermosifiliogr. 2019;110:554–560.