Dermatofibroma is one of the most common benign skin tumors. It typically develops on the lower limbs between the third and fifth decade of life and is more common in women. Clinical diagnosis is often straightforward. Dermatofibromas are associated with a very low rate of local recurrence following excision.
ObjectivesTo describe the clinical and histologic features of dermatofibroma of the face based on our experience.
Materials and methodsDescriptive retrospective study of the clinicopathologic features of dermatofibromas of the face diagnosed at the dermatology department of Hospital General Universitario de Valencia between 1990 and 2012.
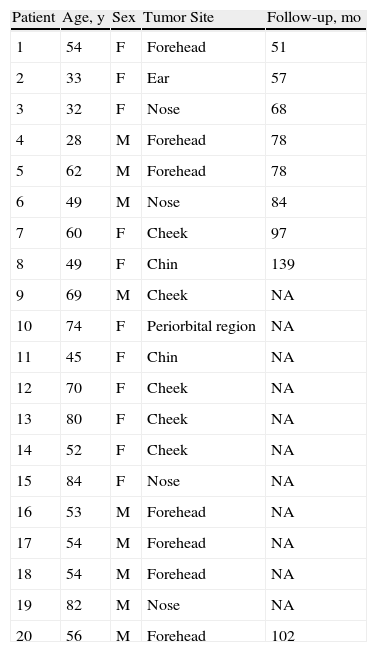
ResultsTwenty cases of dermatofibroma of the face (1.11% of all dermatofibromas diagnosed) were studied. The age at onset varied widely, from 28 to 84 years. The mean age at onset was 57.15 years and the median was 54 years. There were 11 women and 9 men. Mean follow-up was 83 months and there were no local recurrences. All the tumors were confined to the papillary and reticular dermis and the storiform pattern was the most common growth pattern observed.
ConclusionsThis study of facial dermatofibromas diagnosed at our hospital over a period of 22 years suggests that the face is an uncommon site but that dermatofibromas in this location behave similarly to those occurring elsewhere on the body.
El dermatofibroma es uno de los tumores cutáneos benignos más frecuentes. Suele aparecer en las extremidades inferiores entre la tercera y la quinta décadas de la vida, siendo más frecuente en mujeres. El diagnóstico clínico frecuentemente es sencillo. Se asocia a una tasa muy baja de recidivas locales tras la extirpación.
ObjetivosPresentar nuestra experiencia en dermatofibromas de localización facial con el fin de discutir las características clínicas e histopatológicas en esta localización.
Material y métodosEstudio retrospectivo descriptivo de las características clínico-patológicas de los dermatofibromas de localización facial diagnosticados en el Departamento de Dermatología del Hospital General Universitario de Valencia entre los años 1990 y 2012.
ResultadosSe incluyeron 20 casos de dermatofibromas de localización facial (1,11% de los diagnosticados en todas las localizaciones). Estas lesiones mostraron un amplio rango de edad de aparición, que osciló de 28 a 84 años, con una media de 57,15 años y una mediana de 54 años. La distribución por sexo fue de 11 mujeres y 9 hombres. El promedio de seguimiento fue de 83 meses, con ninguna recurrencia local. Todos los casos estaban confinados en la dermis papilar y reticular, y el patrón de crecimiento predominante fue el estoriforme.
ConclusionesEl estudio de los dermatofibromas de localización facial observados en nuestro centro en un período de 22 años sugiere que esta es una localización infrecuente, pero que en la mayoría de los casos tiene un comportamiento similar al de otras localizaciones.
Dermatofibroma, also known as benign fibrous histiocytoma, is one of the most common benign skin tumors. It typically develops on the lower limbs between the third and fifth decade of life, but it can occur at any age. It is more common in women. The tumor presents as a hard, asymptomatic, slow-growing papule or nodule varying in color from a reddish-brown to a violaceous blue. Clinical diagnosis is generally straightforward. Dermatofibroma is associated with a very low rate of local recurrence after excision and metastasis is rare. Several morphologic variants have been reported in the literature, including atrophic dermatofibroma,1 pseudosarcomatous dermatofibroma,2 granular cell dermatofibroma,3 clear cell dermatofibroma,4 epithelioid fibrous histiocytoma,5 dermatofibroma with smooth-muscle proliferation,6 dermatofibroma with myofibroblastic proliferation,7 and palisading cutaneous fibrous histiocytoma.8 Certain histologic variants (cellular,9 aneurysmal,10 and pseudosarcomatous11) are associated with a high rate of local recurrence and, in very rare cases, metastasis.
In recent years there have been reports of dermatofibromas of the face that tend to involve deeper structures and have a higher rate of local recurrence than dermatofibromas on the lower limbs; the observations have prompted the recommendation that dermatofibromas of the face should be excised with wider surgical margins.12 The aim of the present study was to describe the clinical and histopathologic features of dermatofibroma of the face based on our experience.
Material and MethodsWe studied all cases of dermatofibroma diagnosed at the dermatology department of Hospital General Universitario de Valencia in Valencia, Spain between 1990 and 2012 and selected those located on the face. We made a note of the clinical diagnosis mentioned on the biopsy request form, as well as the patient's age and sex and the exact location of the tumor. In all cases, the tissue was fixed in 4% buffered formalin and embedded in paraffin. Sections measuring 4 μm were cut and stained with hematoxylin and eosin. In equivocal cases, paraffin blocks were sectioned for immunohistochemical staining with CD34 (prediluted, qbend/1, Leica Microsystems), S-100 protein (prediluted, Leica Microsystems), and factor xiiia (Clone E980.1, Leica Microsystems). Appropriate positive and negative controls were included in each run. The mean number of mitotic figures per 10 high-power fields (1 high-power field=0.159mm2 on the microscope used) was calculated in each case.
ResultsThe clinical characteristics of the patients are summarized in Table 1. The age of the patients (11 women and 9 men; ratio, 1.22:1) at the time of diagnosis ranged from 28 to 84 years (mean, 57.15 years; median, 54 years). Seven of the 20 dermatofibromas were located on the forehead, 5 on the cheek, 4 on the nose, 2 on the chin, 1 on the ear, and 1 in the periorbital region. The tumors presented as papules or nodules that were firm on palpation. A clinical diagnosis of dermatofibroma had been established in just 2 cases. In the other cases, the suspected diagnosis had been epidermal cyst (n=4), basal cell carcinoma (n=2), fibrous papule (n=2), fibrokeratoma (n=1), hidrocystoma (n=1), intradermal melanocytic nevus (n=2), and adnexal tumor (n=1). There was no mention of a suspected diagnosis In the remaining 5 cases.
Clinical Findings in 20 Cases of Dermatofibroma of the Face.
| Patient | Age, y | Sex | Tumor Site | Follow-up, mo |
| 1 | 54 | F | Forehead | 51 |
| 2 | 33 | F | Ear | 57 |
| 3 | 32 | F | Nose | 68 |
| 4 | 28 | M | Forehead | 78 |
| 5 | 62 | M | Forehead | 78 |
| 6 | 49 | M | Nose | 84 |
| 7 | 60 | F | Cheek | 97 |
| 8 | 49 | F | Chin | 139 |
| 9 | 69 | M | Cheek | NA |
| 10 | 74 | F | Periorbital region | NA |
| 11 | 45 | F | Chin | NA |
| 12 | 70 | F | Cheek | NA |
| 13 | 80 | F | Cheek | NA |
| 14 | 52 | F | Cheek | NA |
| 15 | 84 | F | Nose | NA |
| 16 | 53 | M | Forehead | NA |
| 17 | 54 | M | Forehead | NA |
| 18 | 54 | M | Forehead | NA |
| 19 | 82 | M | Nose | NA |
| 20 | 56 | M | Forehead | 102 |
Abbreviations: F, female; M, male; NA, not available.
In total, 1801 dermatofibromas were diagnosed during the period analyzed. We reviewed 30 biopsies from the database that reported a histologic diagnosis of dermatofibroma of the face. Ten of these were excluded: 8 because the histologic diagnosis did not correspond to the diagnosis recorded in the database and 2 because there were no slides available. We therefore studied 20 cases of facial dermatofibroma (1.1% of all dermatofibromas in the database).
All the patients were treated with local excision with minimal margins. Eleven patients had been lost to follow-up or had died of other causes, so follow-up information was available for just 9 patients. The mean follow-up time was 83 months (median 6.5 years; range, 51 months-11 years). There was no record of recurrences or metastases, and there were no dermatofibroma-related deaths.
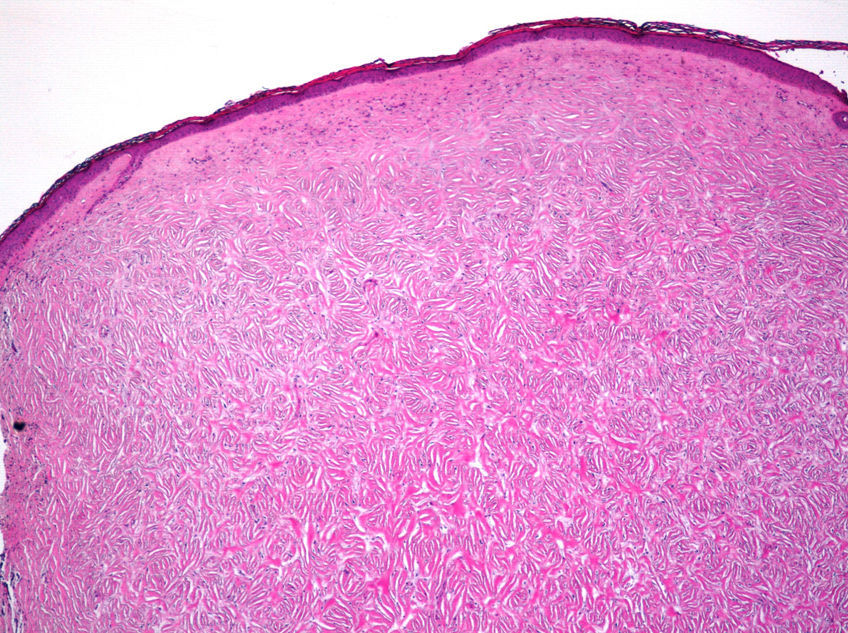
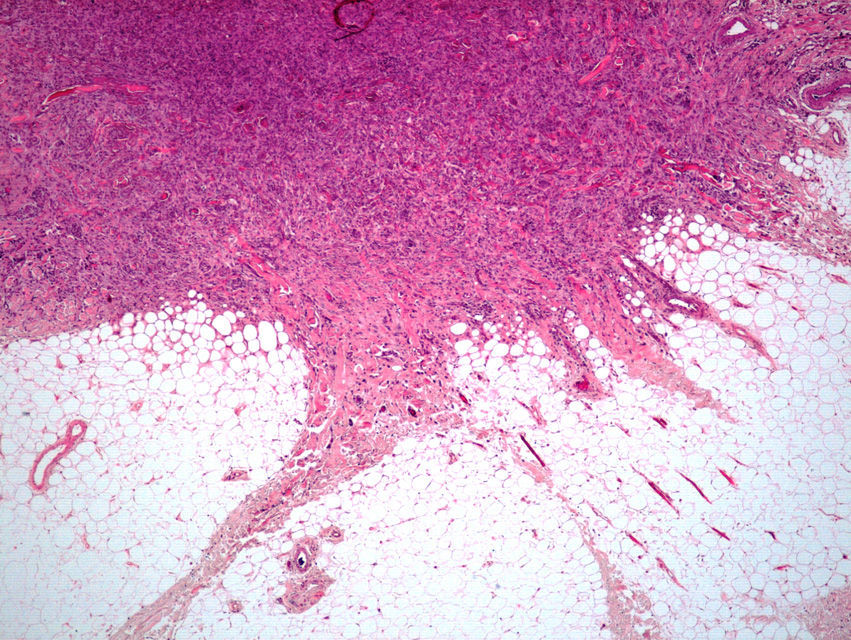
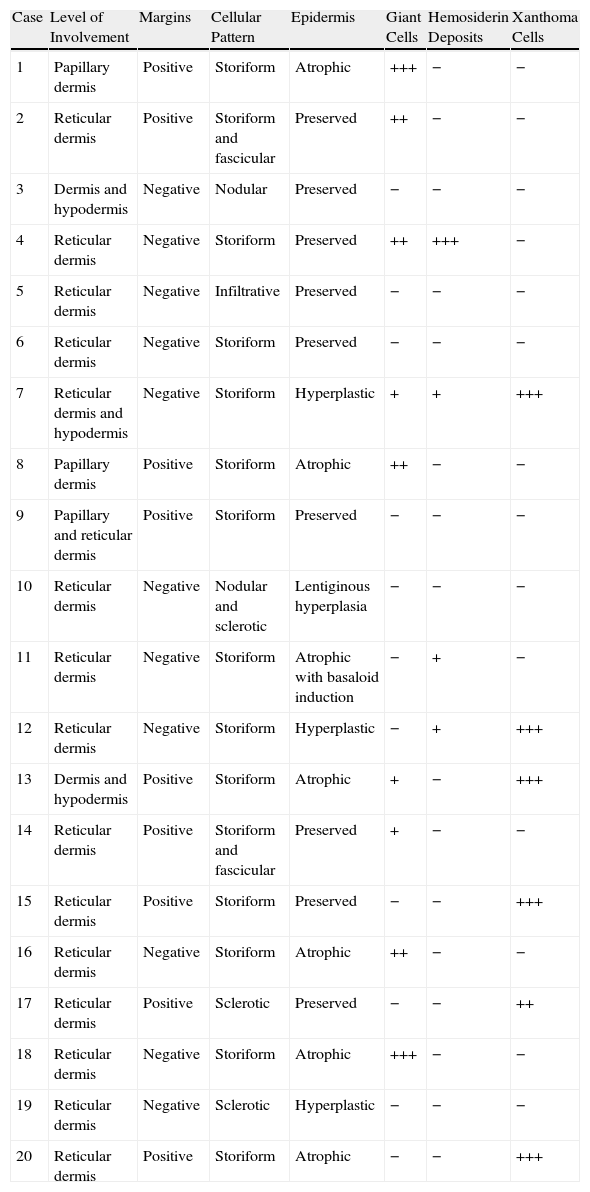
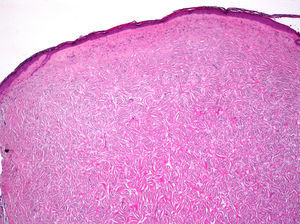
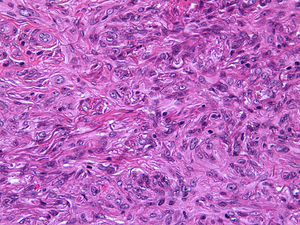
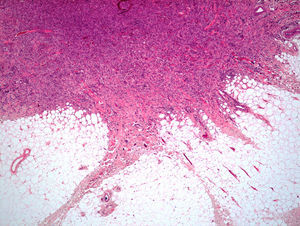
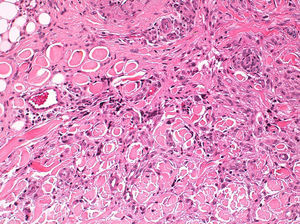
Histopathologic FindingsAll 20 tumors were located in the papillary and reticular dermis, and focal involvement of the subcutaneous tissue was observed in just 3 cases. The surgical margins were positive in 9 tumors, and based on the information available, there were no recurrences. In 7 cases, the epidermis was atrophic, and in 1 of these there was minimal basaloid induction. Epidermal hyperplasia was seen in 4 cases and the epidermis was preserved in 9. A storiform growth pattern was observed in 15 cases and in 2 of these it was combined with fascicles of spindle cells. The pattern was nodular in 3 tumors (with sclerotic areas in 1 case) and sclerotic in 2.
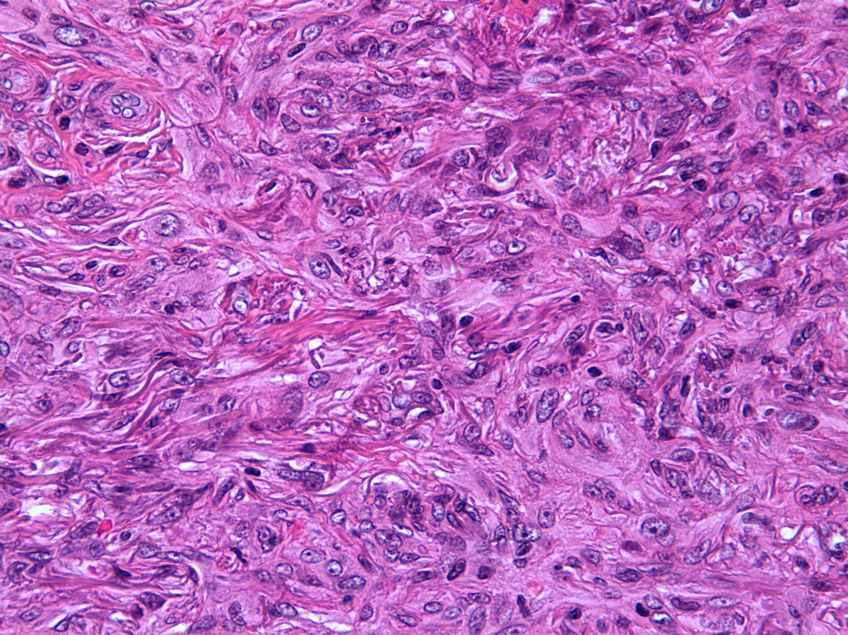
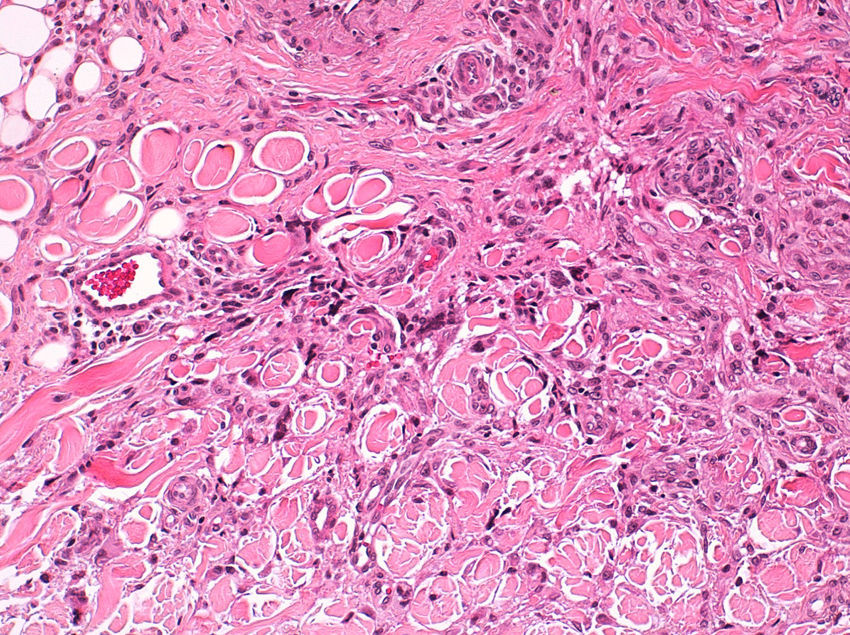
Seventeen tumors had a slight inflammatory infiltrate while the other 3 had a predominantly plasmacytic infiltrate. Histiocytes filled with cholesterol esters (xanthomatous cells) were seen in 6 cases. Hemosiderin deposits were observed in 4 tumors, but they were intense in just 1 of these. Giant cells (focal in 3 cases and intense in 2) were seen in 9 tumors. In 15 cases, the hyalinized collagen bundles in the dermal portion at the periphery of the lesion were surrounded by tumor cells. No mitotic figures were observed in any of the high-power fields analyzed. There was also no evidence of tumor necrosis, vascular invasion, or infiltration of the striated muscle (Figs. 1-4). The histology findings are summarized in Table 2.
Histologic Findings in 20 Cases of Dermatofibroma of the Face.
| Case | Level of Involvement | Margins | Cellular Pattern | Epidermis | Giant Cells | Hemosiderin Deposits | Xanthoma Cells |
| 1 | Papillary dermis | Positive | Storiform | Atrophic | +++ | − | − |
| 2 | Reticular dermis | Positive | Storiform and fascicular | Preserved | ++ | − | − |
| 3 | Dermis and hypodermis | Negative | Nodular | Preserved | − | − | − |
| 4 | Reticular dermis | Negative | Storiform | Preserved | ++ | +++ | − |
| 5 | Reticular dermis | Negative | Infiltrative | Preserved | − | − | − |
| 6 | Reticular dermis | Negative | Storiform | Preserved | − | − | − |
| 7 | Reticular dermis and hypodermis | Negative | Storiform | Hyperplastic | + | + | +++ |
| 8 | Papillary dermis | Positive | Storiform | Atrophic | ++ | − | − |
| 9 | Papillary and reticular dermis | Positive | Storiform | Preserved | − | − | − |
| 10 | Reticular dermis | Negative | Nodular and sclerotic | Lentiginous hyperplasia | − | − | − |
| 11 | Reticular dermis | Negative | Storiform | Atrophic with basaloid induction | − | + | − |
| 12 | Reticular dermis | Negative | Storiform | Hyperplastic | − | + | +++ |
| 13 | Dermis and hypodermis | Positive | Storiform | Atrophic | + | − | +++ |
| 14 | Reticular dermis | Positive | Storiform and fascicular | Preserved | + | − | − |
| 15 | Reticular dermis | Positive | Storiform | Preserved | − | − | +++ |
| 16 | Reticular dermis | Negative | Storiform | Atrophic | ++ | − | − |
| 17 | Reticular dermis | Positive | Sclerotic | Preserved | − | − | ++ |
| 18 | Reticular dermis | Negative | Storiform | Atrophic | +++ | − | − |
| 19 | Reticular dermis | Negative | Sclerotic | Hyperplastic | − | − | − |
| 20 | Reticular dermis | Positive | Storiform | Atrophic | − | − | +++ |
Symbols: +, sparse; ++, moderate; +++, intense; −, none.
An immunohistochemical study was requested in 5 cases to aid the differential diagnosis. Two of the tumors stained for factor xiiia (cases 1 and 9), 1 was negative for S-100 protein (case 2) and 2 were negative for CD34 (cases 13 and 17).
DiscussionDermatofibroma, or benign fibrous histiocytoma, is one of the most common fibrohistiocytic proliferations encountered in daily clinical practice. Its exact line of differentiation and its tumoral13 and reactive14 nature have been widely discussed. The fact that dermatofibroma can develop after minor trauma or an insect bite suggests a reactive origin, while the demonstration of cytogenetic abnormalities and clonality and the possibility of metastasis to the lymph nodes15 and distant organs16 supports the theory that dermatofibroma is a truly neoplastic disease. It originates from dermal dendritic cells of monocyte/macrophage lineage.14,17
Clinically, dermatofibroma typically presents as a small pigmented papule or nodule on the lower limbs of young adults; local recurrence, even in cases of incomplete excision, is observed in less than 1% of cases.18 Histologically, dermatofibroma typically presents as a poorly circumscribed dermal tumor, with underlying epidermal hyperplasia, basal hyperpigmentation, and elongated interpapillary crests that do not generally extend as far as the subcutaneous tissue. It is formed by spindle-cell fascicles, foamy histiocytosis, and giant multinucleated cells, in addition to lymphocytes interspersed in a myxoid stroma with a variable proportion of blood vessels. Entrapment of hyalinized collagen bundles by tumor cells may also been seen at the periphery of the lesion, in addition to a variable inflammatory infiltrate and on occasions foci of hemorrhage. The degree of hyalinization and hemosiderin deposition depends on how long the lesion has been present. Basaloid induction and epidermal hyperplasia represent an autocrine or paracrine reparative response mediated by epidermal growth factor.19
The face is an uncommon site for dermatofibroma. In our series, only 2 cases of dermatofibroma of the face were suspected clinically. This low rate of clinical diagnosis is consistent with reports in the literature, where a majority of facial dermatofibromas are not initially suspected. The most common diagnoses proposed are basal cell carcinoma, adnexal tumor, epidermal cyst, melanocytic nevus, and cutaneous lymphoma.
There have been reports of more aggressive behavior in dermatofibromas of the face than in dermatofibromas at other sites, despite similar clinical and histologic findings. In one series, recurrence was observed in 4 out of 34 patients with facial dermatofibroma.20 There have, however, also been isolated reports of no recurrences in patients followed for years.21–24 There has also been a report of an aggressive intraoral dermatofibroma.25
The possible aggressive nature of dermatofibroma of the face has been linked to deeper infiltration (with occasional involvement of striated muscle, which is located closer to the surface on the face than at other sites), a moderate mitotic rate, and cellular atypia. Although histologic evidence of mitotic activity is a cause for concern, no large series of dermatofibroma have found an association between mitotic rate and recurrence. In one series of 4 patients with 4 or more mitoses per 10 high-power fields, none of the patients developed local recurrence or distant metastasis in 71 months of follow-up, and in another26 series of 34 patients, histopathologic features did not predict the clinical behavior of dermatofibromas of the face.20 There were 4 local recurrences: 2 involving the striated muscle and 2 involving the subcutaneous tissue. Atypia and mitotic rate were not found to be correlated with recurrence in any of the cases.
Dermatofibromas arising on the face should be excised with wide margins as they tend to extend into deep layers of the dermis and hypodermis. In the case of dermatofibromas of the lower limbs, by contrast, local excision, even if incomplete, is not associated with a higher rate of recurrence. In our series, all the dermatofibromas were excised with minimal margins and we detected no evidence of recurrence.
The presence of spindle cells expressing α-smooth muscle actin, seen in cellular dermatofibroma, has also been associated with greater local aggressiveness. The variants associated with the highest rate of local recurrence are cellular and pseudosarcomatous dermatofibroma. Cellular dermatofibroma is characterized by the presence of round, angular epithelioid cells with abundant eosinophilic cytoplasm immersed in a fibrous stroma.9 Pseudosarcomatous dermatofibroma is an uncommon variant that generally arises on the trunk or the limbs of young adults. Histologically, it is characterized by the presence of a nonencapsulated tumor in the dermis or hypodermis, occasionally separated from the epidermis by a Grenz zone. It is composed of histiocytes, multinucleated giant cells and fibroblasts, numerous capillaries, and atypical or monstruous cells with foamy cytoplasm, a hyperchromatic nucleus, and 1 or more prominent nucleoli. The degree of atypia is variable and mitotic activity is low.11 In a series of 7 metastatic dermatofibromas, there were 4 cases of cellular dermatofibroma, 1 case of aneurysmal dermatofibroma, 1 case of pseudosarcomatous dermatofibroma, and 1 case of classical dermatofibroma.27 Factors associated with metastasis include high cellularity, aneurysmal changes, marked cellular pleomorphism, high mitotic activity, tumor necrosis, and repeated local recurrences.27 None of these features were observed in our series.
Dermatofibroma of the face must be distinguished both clinically and histologically from dermatofibrosarcoma protuberans (DFSP), malignant fibrous histiocytoma, and plaque-like CD34-positive dermal fibroma. DFSP is histologically characterized by a proliferation of monomorphic spindle cells with uniformly distributed chromatin arranged in fascicles forming a storiform pattern and diffuse infiltration of the dermal stroma with frequent involvement of the subcutaneous fat; the cells stain positively for CD34.28 Malignant fibrous histiocytoma is a sarcoma that histologically exhibits spindle cells that are reminiscent of fibroblasts and pleomorphic areas with histiocyte-like cells. Immunhistochemistry is strongly positive for vimentin in neoplastic cells.29 Plaque-like CD34-positive dermal fibroma tends to appear as a solitary indurated lesion that may exhibit atrophy. Histology findings include a proliferation of horizontally arranged spindle-shaped cells in the superficial dermis, with superficial cells arranged perpendicular to the epidermis and cells in deeper layers arranged horizontally.30
In conclusion, dermatofibroma of the face is diagnostically challenging, as given its low incidence, it is not often included in the clinical differential diagnosis. Contrasting with reports in the literature, in our series of dermatofibromas of the face we did not observe a diffuse infiltration pattern, involvement of deep structures, cell atypia, or evidence of mitosis. The predominant histologic pattern was similar to that seen in dermatofibromas in other areas, namely a storiform growth pattern and fascicles of spindle cells.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Estela J.R, Rico M.T, Pérez A, Unamuno B, Garcías J, Cubells L, Alegre V. Dermatofibromas faciales: estudio clínico-patológico de 20 casos. Actas Dermosifiliogr. 2014;105:172–177.